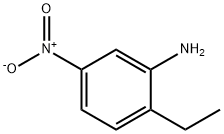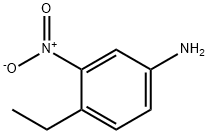
4-ETHYL-3-NITROANILINE synthesis
- Product Name:4-ETHYL-3-NITROANILINE
- CAS Number:51529-96-5
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18
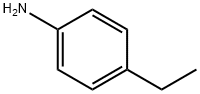
589-16-2

51529-96-5
Step 1: 4-Ethylaniline (10.3 mL, 82.5 mmol) was slowly added dropwise to 96% sulfuric acid (63 mL), and the reaction system was cooled to 8°C and ensured that the temperature was maintained below 10°C. After the dropwise addition was completed, the reaction mixture was further cooled to -5 °C, followed by the slow addition of a mixed acid consisting of 100% nitric acid (4 mL) and 96% sulfuric acid (10 mL), controlling the reaction temperature below 0 °C. The reaction mixture was stirred continuously at -5°C for 1 hour. Upon completion of the reaction, the mixture was slowly poured into ice water (200 mL), precipitated, filtered and washed with water. The resulting solid was resuspended in water (100 mL) and neutralized to neutral with 35% ammonium hydroxide solution. The precipitate was collected by filtration and dried in an oven to give the light brown solid product 4-ethyl-3-nitroaniline (10 g, 73% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 1.11 (t, J = 7.45 Hz, 3H), 2.63 (q, J = 7.45 Hz, 2H), 5.53 (s, 2H), 6.81 (dd, J = 8.30, 2.44 Hz, 1H), 7.04 (d, J = 2.44 Hz, 1H), 7.11 ( d, J = 8.30 Hz, 1H).

589-16-2
228 suppliers
$10.00/1g

51529-96-5
40 suppliers
inquiry
Yield:51529-96-5 73%
Reaction Conditions:
Stage #1: 4-aminoethylbenzenewith sulfuric acid;HNO3 at -5 - 10; for 1 h;
Stage #2: with ammonium hydroxide in lithium hydroxide monohydrate;
Steps:
K.1
Step 1 : 4-Ethyl-3-nitroaniline4-Ethylaniline (10.3 mL, 82.5 mmol) was added dropwise to sulfuric acid (96%, 63 mL), cooled to 8 °C, maintaining the temperature below 10 °C. After the addition, the reaction mixture was cooled to -5 °C, before the addition of a mixture of nitric acid (100%, 4 mL) and sulfuric acid (96%, 10 mL), keeping the temperature below 0 °C. The reaction mixture was then stirred at the same temperature for 1 h. The reaction mixture was poured into ice (200 mL) and the precipitate filtered and washed with water. The solid was suspended with water (100 mL) and neutralized with ammonium hydroxide (35%). The precipitate was filtered and dried in the oven to obtain a light-brown solid (10 g, 73%).1H NMR (400 MHz, DMSO-d6) δ ppm 1.11 (t, J =7.45 Hz, 3 H), 2.63 (q, J =7.45 Hz, 2 H), 5.53 (s, 2 H), 6.81 (dd, J =8.30, 2.44 Hz, 1 H), 7.04 (d, J =2.44 Hz, 1 H), 7.11 (d, J =8.30 Hz, 1 H)
References:
WO2012/139930,2012,A1 Location in patent:Page/Page column 72
5805-89-0
1 suppliers
inquiry

51529-96-5
40 suppliers
inquiry
1204-29-1
6 suppliers
inquiry

51529-96-5
40 suppliers
inquiry

3663-34-1
27 suppliers
$106.00/50mg

51529-96-5
40 suppliers
inquiry