
4-FLUORO-2-METHOXYBENZAMIDE synthesis
- Product Name:4-FLUORO-2-METHOXYBENZAMIDE
- CAS Number:874804-07-6
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
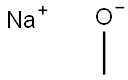
124-41-4
671 suppliers
$12.00/25g
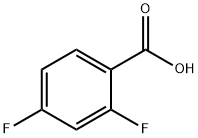
1583-58-0
410 suppliers
$6.00/5g

874804-07-6
28 suppliers
$317.00/10g
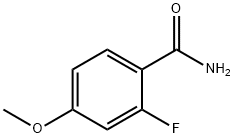
874804-31-6
23 suppliers
$45.00/50mg
Yield: 2% , 57%
Reaction Conditions:
Stage #1:sodium methylate;2,4-difluoro-benzoic acid in dimethyl sulfoxide at 80; for 25 h;
Stage #2: with thionyl chloride;N,N-dimethyl-formamide for 4 h;Reflux;
Stage #3: with ammonium hydroxide in dichloromethane;water at 20;Cooling;
Steps:
92.1
Step 1
15.81 g 2,4-Difluorobenzoic acid (100 mmol) was dissolved in 200 ml DMSO and 10.80 g sodium methoxyide (200 mmol) was added.
The mixture was heated at 80 C for 25 hours.
Then it was poured onto 2000 g ice and pH was set to 1-2 by addition of saturated HCl solution.
The precipitated solid was collected by filtration and dried under vacuum over phosphorus pentoxide for a day.
The dry white solid was refluxed in 150 ml thionyl chloride in presence of 2-3 drops of dry DMF for 4 hours.
Volatiles were removed by evaporation, toluene was added to the residue and it was evaporated off again.
Finally the residue was taken up in 150 ml dry dichloromethane and was added dropwise to 300 ml cooled solution of 12% NH4OH in water.
After the addition the mixture was stirred for an hour at room temperature, then it was extracted four times with 300-300 ml chloroform.
The combined organic layer was washed with brine, dried over MgSO4 and evaporated to dryness.
The residue was purified by column chromatography on silica gel eluting with hexanes:ethyl acetate=1:1.
2-Fluoro-4-methoxy-benzamide was obtained as a white solid. Yield: 0.35 g (2%). Ret. time: 2.21 min., (M+H)+=170; 1HNMR (DMSO-d6, 300 MHz), δ (ppm): 7.68 (t, J=8.67 Hz, 1H), 7.42 (bd, 2H), 6.86 (m, 2H), 3.81 (s, 3H).
4-Fluoro-2-methoxy-benzamide was obtained as a white solid. Yield: 9.66 g (57%). Ret. time: 2.36 min., (M+H)+=170; 1HNMR (DMSO-d6, 300 MHz), δ (ppm): 7.86 (t, J=7.97 Hz, 1H), 7.54 (bd, 2H), 7.03 (dd, J3=11.40 Hz, J4=2.31 Hz, 1H), 6.85 (dt, J3=8.46 Hz, J4=2.37 Hz, 1H), 3.90 (s, 3H).
References:
Lori, Franco;Chafouleas, James;De Forni, Davide;Solinas, Antonio;Varga, Zoltán;Greff, Zoltán;Örfi, László;Kéri, György US2014/57911, 2014, A1 Location in patent:Paragraph 0537-0540