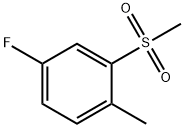
4-Fluoro-2-(Methylsulfonyl)toluene synthesis
- Product Name:4-Fluoro-2-(Methylsulfonyl)toluene
- CAS Number:828270-66-2
- Molecular formula:C8H9FO2S
- Molecular Weight:188.22
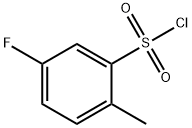
445-05-6
128 suppliers
$12.00/1g

74-88-4
356 suppliers
$15.00/10g

828270-66-2
25 suppliers
$195.00/250mg
Yield:828270-66-2 73%
Reaction Conditions:
Stage #1: 5-fluoro-2-methylbenzenesulfonyl chloridewith hydrazine in tetrahydrofuran at 0; for 16 h;
Stage #2: methyl iodidewith sodium acetate in ethanol; for 16 h;Heating / reflux;
Steps:
32
Following the published method of Grunewald et al. (J. Med. Chem. 1999, 42, 3220) for the conversion of sulfonyl chlorides to the corresponding alkylsulfonates, to 800 mg, 3.8 mmol of the commercially available sulfonyl chloride in 20 mL THF at 0° C. was added 0.5 mL, ca. 2.2 eq of hydrazine. After 16h, the reaction solvent was removed to give a white solid. At this time, the reaction was taken up in 10 mL EtOH, and excess sodium acetate (10 eq) and methyl iodide (5 eq) were added. The reaction was then heated to reflux for 16h. At this time, the reaction was concentrated and the residue chromatographed on silica gel (4:1 hexanes/EtOAc) to give 0.4 g of (73%) pure product.
References:
US2007/72831,2007,A1 Location in patent:Page/Page column 83

67-56-1
790 suppliers
$9.00/25ml
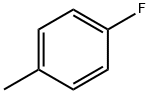
352-32-9
317 suppliers
$9.00/5g

828270-66-2
25 suppliers
$195.00/250mg