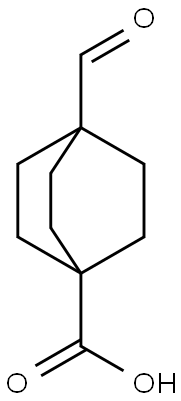
4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid synthesis
- Product Name:4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid
- CAS Number:96102-85-1
- Molecular formula:C10H14O3
- Molecular Weight:182.22
![Sodium hydroxy(4-(methoxycarbonyl)bicyclo-[2.2.2]octan-1-yl)methanesulfonate](/CAS/GIF/1308728-99-5.gif)
1308728-99-5
8 suppliers
inquiry
![4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/96102-85-1.gif)
96102-85-1
23 suppliers
inquiry
Yield:96102-85-1 90%
Reaction Conditions:
with sulfuric acid;acetic acid at 100;Industry scale;Inert atmosphere;
Steps:
3
Preparation of Aldehyde 14 from 13: Under nitrogen, to 11.4 Kg of 13 in 14.9 L of acetic acid was added 29.8 L of 2M H2SO4. The resulting mixture was slowly heated to 100°C and allowed to stir for one hour. Distillation of the acid solvents was carried out until the final volume was 3.5 liters, 3.5 x 11.4 liters. The mixture was then heated to 100°C until the reaction was complete. The mixture was cooled to room temperature and stirred for an additional 10 hours. The mixture was then cooled to 0°C for 3 hours. The resulting suspension is filtered and the filter cake is washed three times with cold water. The wet cake is dried at about 50°C until KF is < 0.1% to give 5.5 kg of 14, 90% molar yield.
References:
WO2011/63268,2011,A2 Location in patent:Page/Page column 38
![4-Formyl-bicyclo[2.2.2]octane-1-carboxylic acid methyl ester](/CAS/20180808/GIF/94994-25-9.gif)
94994-25-9
53 suppliers
inquiry
![4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/96102-85-1.gif)
96102-85-1
23 suppliers
inquiry
![BICYCLO[2.2.2]OCTANE-1,4-DICARBOXYLIC ACID HEMIMETHYL ESTER](/CAS/GIF/18720-35-9.gif)
18720-35-9
245 suppliers
$19.00/1g
![4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/96102-85-1.gif)
96102-85-1
23 suppliers
inquiry
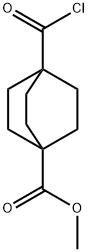
110371-29-4
2 suppliers
inquiry
![4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/96102-85-1.gif)
96102-85-1
23 suppliers
inquiry
![Methyl 4-(hydroxyMethyl)bicyclo[2.2.2]octane-1-carboxylate](/CAS/20150408/GIF/94994-15-7.gif)
94994-15-7
53 suppliers
$54.00/100mg
![4-ForMyl-bicyclo[2.2.2]octane-1-carboxylic acid](/CAS/20150408/GIF/96102-85-1.gif)
96102-85-1
23 suppliers
inquiry