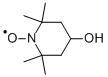
4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy synthesis
- Product Name:4-Hydroxy-2,2,6,6-tetramethyl-piperidinooxy
- CAS Number:2226-96-2
- Molecular formula:C9H18NO2*
- Molecular Weight:172.24
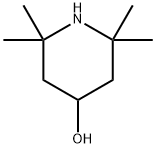
2403-88-5
348 suppliers
$5.00/10g

2226-96-2
503 suppliers
$5.00/5g
Yield:2226-96-2 86%
Reaction Conditions:
with tert.-butylhydroperoxide;hexacarbonyl molybdenum in 1,2-dichloro-ethane;
Steps:
1 Preparation of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl
EXAMPLE 1 Preparation of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl A reactor is charged with 50 ml of reagent-grade 1,2-dichloroethane, 8.5 g (0.054 mole) of 2,2,6,6-tetramethylpiperidin-4-ol and 0.10 g of molybdenum hexacarbonyl, Mo(CO)6. The mixture is brought to reflux to give a clear solution. A dropping funnel is charged with 27 ml of 4M tert-butyl hydroperoxide in 1,2-dichloroethane and this solution is then added dropwise into the reactor, at a rate sufficient to maintain a gentle reflux without external heat. The addition requires about 0.5 hour after which heat is applied for 4 hours. At this point gas chromatography (GC) showed <2% of unreacted amine. The reaction mixture is cooled, washed with 5% aqueous sodium sulfite. The aqueous phase is extracted with 50 ml of chloroform and the combined organic phases are dried over anhydrous magnesium sulfate. After evaporation of the solvents, the residue is crystallized from hexane, yielding 8.0 g (86% yield) of orange crystals, m.p. 69°-71° (lit. 71° C.) (H. Lemaire, et al, Bull. Soc. Chim. France 1968, 886.).
References:
US4665185,1987,A