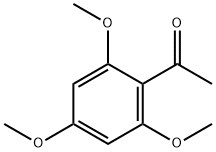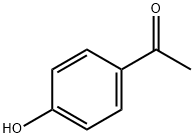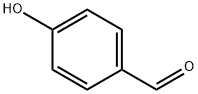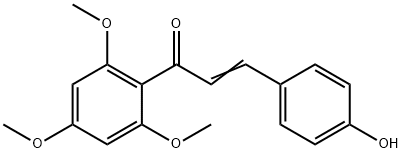
4-Hydroxy-2',4',6'-trimethoxychalcone synthesis
- Product Name:4-Hydroxy-2',4',6'-trimethoxychalcone
- CAS Number:61777-22-8
- Molecular formula:C18H18O5
- Molecular Weight:314.33
Yield:61777-22-8 79%
Reaction Conditions:
with sodium hydroxide in methanol at 20;
Steps:
2.1. general procedures for synthesis of FKd
General procedure: In 20 mL of MeOH, corresponding acetophenones (1.5 mmol, 2-hydroxy-4,6-dimethoxyacetophenone for compounds 1-10, and 2,4,6-trimethoxy-2-hydroxyacetophenone for compounds 11-22),benzaldehyde derivatives (1.5 mmol) and NaOH (2.5 equivalentof acetophenone) were dissolved. The reaction mixture was stirred at room temperature until the completion of the reaction which was monitored by TLC. The excess of NaOH was neutralized by addition of HCl 0.1 M. Compounds 6, 7 and 19 were directly purified by Sephadex column chromatography. For the others, MeOH was evaporated and the solid residue was dissolved in CH2Cl2(50 mL) and washed with distilled water (3 50 mL). The organic phase was dried with MgSO4, filtered and CH2Cl2 was removed under vacuum. Finally, FKd were purified by column chromatography or by crystallization in MeOH or EtOAc. Purity of FKd reached at least 95%, as confirmed by HPLC. All chemicals were purchased from Sigma-Aldrich (Saint-Louis, MO, USA) and HPLC quality solvents from Fischer Chemicals (Leicestershire, UK). Mass spectral(MS) analysis was carried on a Bruecker Esquire HCT Ultra MSinstrument equipped with an electrospray ion source. High resolution mass spectral (HRMS) analysis was carried on a Waters SYNAPT G2 HDMS instrument equipped with an atmospheric pressure ionization source. NMR analyses were performed on a Bruecker Avance III spectrometer. Melting points were determinedon an Electrothermal 9100 apparatus.
References:
Thieury, Charlotte;Lebouvier, Nicolas;Le Guével, Rémy;Barguil, Yann;Herbette, Ga?tan;Antheaume, Cyril;Hnawia, Edouard;Asakawa, Yoshinori;Nour, Mohammed;Guillaudeux, Thierry [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 6,p. 1817 - 1829]