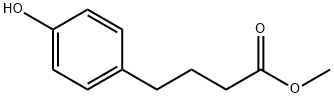
4-Hydroxybenzenebutyric acid methyl ester synthesis
- Product Name:4-Hydroxybenzenebutyric acid methyl ester
- CAS Number:22320-10-1
- Molecular formula:C11H14O3
- Molecular Weight:194.23
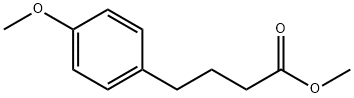
20637-08-5
27 suppliers
$15.00/250mg

22320-10-1
10 suppliers
inquiry
Yield:22320-10-1 98%
Reaction Conditions:
with boron tribromide in dichloromethane at 0;Inert atmosphere;
Steps:
1 4-(4-Hydroxy-phenyl)-butyric acid methyl ester (3)
BBr3 (80 mL, 1.0 M solution in dichloromethane) was added dropwise to a cooled (0° C.) solution of methyl 4-(4-methoxyphenyl)butyrate (2) (10.34 g, 0.05 mol) in dried CH2Cl2 (60 mL) under Ar. After stirring for an additional hour at 0° C., the reaction mixture was treated with 1:1 CH3OH: CH2Cl2 (80 mL) with cooling and stirred overnight at ambient temperature. Concentration of the mixture gave oil, which portioned between ethyl acetate (100 ml) and water (100 ml). The aqueous layer was extracted with EtOAc (2*50 mL), and the combined organic extracts washed with water (50 mL), brine (50 mL), dried over Na2SO4, then concentrated to give the desired phenol as an oil 9.88 g which was purified by flash chromatography (8:2 hexanes/EtOAc) afforded 3 (9.19 g, 98%) as pale yellow oil. 1H NMR (CDCl3) δ 7.01 (d, 2H), 6.80 (d, 2H), 3.68 (s, 3H), 2.55 (t, 2H), 2.34 (t, 2H), 1.93 (t, 2H); 13C NMR (CDCl3) δ 174.8, 154.0, 132.8, 129.3, 115.1, 51.6, 34.0, 33.5, 26.5.
References:
US2019/77774,2019,A1 Location in patent:Paragraph 0316

67-56-1
782 suppliers
$9.00/25ml
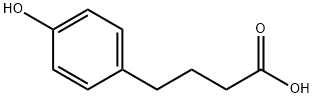
7021-11-6
97 suppliers
$24.00/250mg

22320-10-1
10 suppliers
inquiry

7021-11-6
97 suppliers
$24.00/250mg

74-88-4
356 suppliers
$15.00/10g

22320-10-1
10 suppliers
inquiry
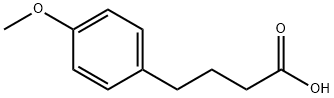
4521-28-2
194 suppliers
$9.00/1g

22320-10-1
10 suppliers
inquiry