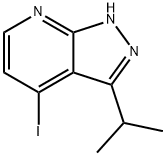
4-iodo-3-isopropyl-1H-pyrazolo[3,4-b]pyridine synthesis
- Product Name:4-iodo-3-isopropyl-1H-pyrazolo[3,4-b]pyridine
- CAS Number:1260539-00-1
- Molecular formula:C9H10IN3
- Molecular Weight:287.1
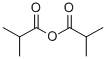
97-72-3
231 suppliers
$10.00/10g
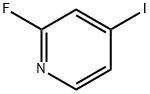
22282-70-8
274 suppliers
$8.00/1g
![4-iodo-3-isopropyl-1H-pyrazolo[3,4-b]pyridine](/CAS/20200331/GIF/1260539-00-1.gif)
1260539-00-1
11 suppliers
inquiry
Yield:1260539-00-1 42%
Reaction Conditions:
Stage #1: 2-fluoro-4-iodopyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 0.25 h;Inert atmosphere;
Stage #2: 2-Methylpropionic anhydride in tetrahydrofuran;hexane at -78; for 1 h;Inert atmosphere;
Stage #3: with hydrazine hydrate in tetrahydrofuran;hexane at 60; for 1 h;
Steps:
100.100.1
Example 100(1)4-Iodo-3-isopropyl-1H-pyrazolo[3,4-b]pyridine (100a)Normal-butyllithium (a 2.6 M solution in hexane, 41.0 mL) was dropwise added to a solution of N,N-diisopropylamine (16.5 mL) in tetrahydrofuran (hereinafter referred to as THF, 300 mL) under a nitrogen atmosphere at -5 to 0° C., and a solution of 2-fluoro-3-iodo-pyridine (24 g) in THF (200 mL) was dropwise added to the resulting mixture at -78° C., followed by stirring for 15 min. Subsequently, isobutyric anhydride (20.0 mL) was dropwise added to the reaction solution at -78° C., followed by stirring at the same temperature for 1 hr, and then hydrazine monohydrate (10.4 mL) was added to the reaction solution, followed by stirring at 60° C. for 1 hr. The reaction solution was distributed between ethyl acetate and water, and the organic layer was washed with saturated saline. The organic layer after the washing was dried over anhydrous sodium sulfate, and then the solvent was distilled away. The residue was purified by neutral silica gel column chromatography (hexane/ethyl acetate) to obtain compound (100a) (12.9 g, 42%) as a white solid.1H-NMR (CDCl3) δ: 12.02 (1H, brs), 8.11 (1H, d, J=4.88 Hz), 7.63 (1H, d, J=4.88 Hz), 3.93 (1H, tt, J=6.83, 6.83 Hz), 1.48 (6H, d, J=6.83 Hz); LRMS (ESI) m/z 288 [M+H]+.
References:
US2012/108589,2012,A1 Location in patent:Page/Page column 13

97-72-3
231 suppliers
$10.00/10g
113975-22-7
314 suppliers
$7.00/1g
![4-iodo-3-isopropyl-1H-pyrazolo[3,4-b]pyridine](/CAS/20200331/GIF/1260539-00-1.gif)
1260539-00-1
11 suppliers
inquiry