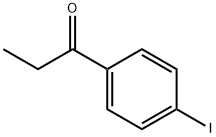
4-iodopropiophenone synthesis
- Product Name:4-iodopropiophenone
- CAS Number:31970-26-0
- Molecular formula:C9H9IO
- Molecular Weight:260.07
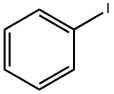
591-50-4
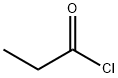
79-03-8

31970-26-0
Iodobenzene (100 g, 0.49 mol) was placed in a dry 1 L three-necked flask fitted with an N2 inlet and 200 mL of carbon disulfide (CS2) was added to it. The reaction mixture was cooled to 0-5 °C, followed by the addition of anhydrous aluminum trichloride (AlCl3, 80 g, 0.6 mol) in batches, followed by the slow dropwise addition of propionyl chloride (60 g, 0.64 mol) while the reaction temperature was controlled to be maintained at 5-10 °C. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the mixture was slowly poured into a 5 L plastic beaker containing 1 L of 10% hydrochloric acid (HCl) and 1 kg of crushed ice. The reaction mixture was extracted with 1 L of ethyl acetate and the organic layer was separated and washed sequentially with 2 x 500 mL of water and 500 mL of saturated saline. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure at 40 °C to give 4'-iodopropiophenone (48 g, 38% yield). The product was characterized by 1H NMR (500 MHz, CDCl3): δ 7.82 (d, 2H), 7.67 (d, J = 8.30 Hz, 2H), 2.96 (q, J = 7.00 Hz, 2H), 1.22 (t, J = 7.32 Hz, 3H).

591-50-4
502 suppliers
$10.00/1g

79-03-8
407 suppliers
$9.00/1g

31970-26-0
45 suppliers
$167.00/250mg
Yield:31970-26-0 38%
Reaction Conditions:
with aluminum (III) chloride in carbon disulfide at 5 - 10; for 24 h;Inert atmosphere;
Steps:
3.A
Iodobenzene (10Og, 0.49 mol) was taken in a dry 1 L 3 neck flask equipped with a N2 inlet and to it 200 ml of CS2 was added. The contents were cooled to 0-50C and then AICI3 (80 g, 0.6 moles) and subsequently propionyl chloride (60 g, 0.64 mol) were added while keeping the temperature (internal) at 5-100C. The contents were stirred for 24 hrs.The reaction mixture was poured into a 5 liter plastic beaker containing 1 L of 10% HCI + 1 Kg of crushed ice. The resultant slurry was extracted with 1 L of ethyl acetate. The organic layer was separated and washed with 2x500 ml of water and 500 ml of brine. The organic layer was dried over sodium sulphate and concentrated at 400C to give 4'-iodopropiophenone (48 g, 38%).1H NMR (500 MHz, CDCI3-d) 7.82 (d, 2H), 7.67 (d, J = 8.30 Hz, 2H), 2.96 (q, J = 7.00 Hz, 2H), 1.22 (t, J = 7.32 Hz, 3H)
References:
WO2010/104488,2010,A1 Location in patent:Page/Page column 62
10342-83-3
225 suppliers
$5.00/5g

31970-26-0
45 suppliers
$167.00/250mg
70-69-9
130 suppliers
$26.00/250mg

31970-26-0
45 suppliers
$167.00/250mg
13183-70-5
136 suppliers
$5.00/250mg

31970-26-0
45 suppliers
$167.00/250mg
![Borane, dichloro[4-(trimethylsilyl)phenyl]-](/CAS/20210305/GIF/107134-72-5.gif)
107134-72-5
0 suppliers
inquiry

31970-26-0
45 suppliers
$167.00/250mg