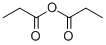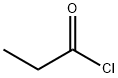
Propionyl chloride synthesis
- Product Name:Propionyl chloride
- CAS Number:79-03-8
- Molecular formula:C3H5ClO
- Molecular Weight:92.52
CH3CH2COOH+PCl3→CH3CH2COCl+HOPCl2
Propionyl chloride is industrially produced by chlorination of propionic acid with phosgene:
CH3CH2CO2H + COCl2 → CH3CH2COCl + HCl + CO2
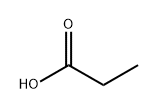
802294-64-0
1 suppliers
inquiry

79-03-8
406 suppliers
$9.00/1g
Yield:79-03-8 97%
Reaction Conditions:
with thionyl chloride in N,N-dimethyl-formamide at 70 - 75;
Steps:
1 First synthesis of propionyl chloride:
First synthesis of propionyl chloride:In a 250 mL three-necked flask equipped with a reflux condenser, propionic acid was added,Then add propionic acid 1.5% dimethylformamide,In the section of the reflux condenser, into the exhaust gas absorption device,Heating up to 70-75 ,Slowly dropping thionyl chloride,The molar ratio of propionic acid to thionyl chloride is 1: 1.5.After the reaction is complete,The excess of thionyl chloride and by-products are removed under reduced pressure,Propionyl chloride,The prepared propionyl chloride was tested to give a colorless propionyl chloride having a purity of more than 98% and a yield of 97.0%
References:
CN106631857,2017,A Location in patent:Paragraph 0056; 0069; 0082
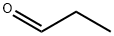
123-38-6
455 suppliers
$14.00/5mL

79-03-8
406 suppliers
$9.00/1g
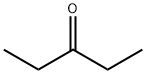
96-22-0
297 suppliers
$9.00/1g
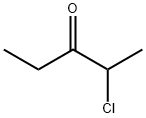
17042-21-6
14 suppliers
inquiry
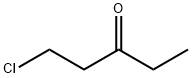
32830-97-0
34 suppliers
$117.00/5g

79-03-8
406 suppliers
$9.00/1g

75-07-0
429 suppliers
$14.00/5mL

96-22-0
297 suppliers
$9.00/1g

17042-21-6
14 suppliers
inquiry

32830-97-0
34 suppliers
$117.00/5g
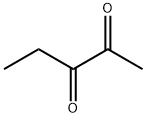
600-14-6
366 suppliers
$11.00/25g

79-03-8
406 suppliers
$9.00/1g

75-07-0
429 suppliers
$14.00/5mL