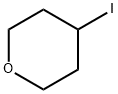
4-IODOTETRAHYDRO-2H-PYRAN synthesis
- Product Name:4-IODOTETRAHYDRO-2H-PYRAN
- CAS Number:25637-18-7
- Molecular formula:C5H9IO
- Molecular Weight:212.03
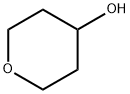
2081-44-9

25637-18-7
GENERAL STEPS: Tetrahydro-2H-pyran-4-ol (2.0 g, 19.58 mmol), imidazole (1.600 g, 23.50 mmol), triphenylphosphine (5.39 g, 20.56 mmol) and tetrahydrofuran (39.2 mL) were added to a round bottom flask (RBF) and the mixture was cooled to 0 °C. A solution of iodine (5.96 g, 23.50 mmol) in tetrahydrofuran (39.2 mL) was slowly added dropwise with stirring. After dropwise addition, the reaction mixture was gradually warmed to room temperature and stirring was continued overnight. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and washed with deionized water. The aqueous layer was back-extracted with ethyl acetate, all organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification of the crude product by column chromatography (using a RediSep Gold 80g silica gel column eluting with a 0-50% ethyl acetate/heptane gradient) afforded 4-iodotetrahydro-2H-pyran (2.67 g, 12.59 mmol, 64.3% yield) as a clarified light yellow oil.

2081-44-9
298 suppliers
$6.00/1g

25637-18-7
171 suppliers
$6.00/250mg
Yield:25637-18-7 64.3%
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in tetrahydrofuran at 0 - 20;
Steps:
Step 1: 4-iodotetrahydro-2H-pyran
A RBF was charged with tetrahydro-2H-pyran-4-ol (2.0 g, 19.58 mmol), imidazole (1.600 g, 23.50 mmol), triphenylphosphine (5.39 g, 20.56 mmol), and tetrahydrofuran (39.2 ml) and cooled to 0° C. A solution of iodine (5.96 g, 23.50 mmol) in tetrahydrofuran (39.2 ml) was added slowly dropwise.
The reaction was warmed to room temperature and stirred overnight.
The reaction was diluted with ethyl acetate and washed with water.
The aqueous layer was extracted with ethyl acetate, and the combined organic layers were dried with sodium sulfate, filtered, and concentrated.
The material was purified via column chromatography (RediSep Gold 80 g, gradient elution 0-50% EtOAc:Heptane) to afford 4-iodotetrahydro-2H-pyran (2.67 g, 12.59 mmol, 64.3% yield) as a clear light yellow oil.
References:
Amgen Inc.;Weiss, Matthew;Boezio, Alessandro;Boezio, Christiane;Butler, John R.;Chu-Moyer, Margaret Yuhua;Dimauro, Erin F.;Dineen, Thomas;Graceffa, Russell;Guzman-Perez, Angel;Huang, Hongbing;Kreiman, Charles;La, Daniel;Marx, Isaac E.;Milgrim, Benjamin Charles;Nguyen, Hanh Nho;Peterson, Emily;Romero, Karina;Sparling, Brian US9212182, 2015, B2 Location in patent:Page/Page column 79
97986-34-0
58 suppliers
$28.00/100mg

25637-18-7
171 suppliers
$6.00/250mg
108-97-4
84 suppliers
$61.00/250mg

25637-18-7
171 suppliers
$6.00/250mg