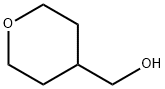
(TETRAHYDRO-2H-PYRAN-4-YL)METHANOL synthesis
- Product Name:(TETRAHYDRO-2H-PYRAN-4-YL)METHANOL
- CAS Number:14774-37-9
- Molecular formula:C6H12O2
- Molecular Weight:116.16
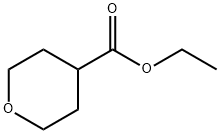
96835-17-5

14774-37-9
Ethyl tetrahydro-2H-pyran-4-carboxylate (5.0 g, 34.7 mmol) was slowly added to a stirred suspension of lithium aluminum hydride (LiAlH4, 4.0 g, 104 mmol) in tetrahydrofuran (THF, 100 mL) at 0 °C. The reaction mixture was kept stirred at 0 °C for 1 hour. Subsequently, ethyl acetate (20 mL) was added slowly and dropwise to the reaction mixture to quench the excess LiAlH4, followed by the addition of 10% aqueous sodium hydroxide (NaOH). The resulting mixture was continued to be stirred for 30 minutes. Upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad to remove insoluble impurities. The filtrate was concentrated under reduced pressure to give the target product (tetrahydro-2H-pyran-4-yl) methanol (9) as a colorless oil. The yield was 3.9 g in 96% yield. The product was characterized by 1H NMR (400 MHz, DMSO-d5): δ 4.46 (broad single peak, 1H), 3.82 (double peak, J=8.0 Hz, 2H), 3.25-3.22 (multiple peaks, 4H), 1.62-1.52 (multiple peaks, 3H), 1.18-1.04 (multiple peaks, 1H).

96835-17-5
86 suppliers
$19.00/1g

14774-37-9
257 suppliers
$5.00/1g
Yield:14774-37-9 96%
Reaction Conditions:
Stage #1: ethyl tetrahydro-2H-pyran-4-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0; for 1 h;
Stage #2: with water;sodium hydroxide in tetrahydrofuran;ethyl acetate; for 0.5 h;
Steps:
1.1
Ethyl tetrahydro-2H-pyran-4-carboxylate (5.0 g, 34.7 mmol) was added to a stirred solution of LiAlH4 (4.0 g, 104 mmol) in THF (100 mE) at 0° C. and the reaction mixture was stirred for 1 h. Ethyl acetate (20 mE) was added dropwise to the reaction mixture followed by addition of 10% aq.NaOH and the resulting mixture was stirred for additional 30 mm. Reaction mass was filtered through Celite and the filtrate was concentrated under reduced pressure to give (tetrahydro-2H-pyran-4-yl)methanol (9) as a colorless oil. Yield (3.9 g, 96%); ‘H NMR (400 MHz, DMSO-d5) δ 4.46 (bs, 1H), 3.82 (dd, J=i0.8, 8.0 Hz, 2H), 3.25-3.22 (m, 4H), 1.62-1.52 (m, 3H), 1.18-1.04 (m, 1H).
References:
US2014/275043,2014,A1 Location in patent:Paragraph 0415; 0416
110238-91-0
215 suppliers
$13.00/5g

14774-37-9
257 suppliers
$5.00/1g
5337-03-1
314 suppliers
$10.00/1g

14774-37-9
257 suppliers
$5.00/1g
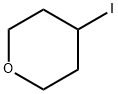
25637-18-7
166 suppliers
$6.00/250mg

50-00-0
896 suppliers
$10.00/25g

14774-37-9
257 suppliers
$5.00/1g
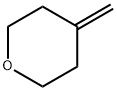
36838-71-8
59 suppliers
$60.00/5g
![9-Borabicyclo[3.3.1]nonane](/CAS/GIF/280-64-8.gif)
280-64-8
153 suppliers
$45.00/50ml

14774-37-9
257 suppliers
$5.00/1g