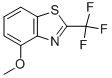
4-METHOXY-2-(TRIFLUOROMETHYL)BENZOTHIAZOLE synthesis
- Product Name:4-METHOXY-2-(TRIFLUOROMETHYL)BENZOTHIAZOLE
- CAS Number:354760-24-0
- Molecular formula:C9H6F3NOS
- Molecular Weight:0
Yield:354760-24-0 44.7 g
Reaction Conditions:
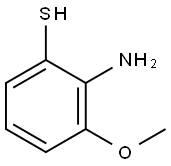
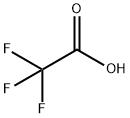
Stage #1: 2-amino-3-methoxybenzenethiol;trifluoroacetic acid at 95; for 2 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
5.2.29. 4-Methoxy-2-(trifluoromethyl)benzo[d]thiazole (40)
A mixture of 39 (50.0 g, 277 mmol) in 1 mol/L KOH aq (275 mL, 275 mmol) was stirred for 25 h under reflux conditions under an argon atmosphere. Ice water was added and the mixture was washed with CH2Cl2. The aqueous layer was acidified with conc. HCl (to approximately pH 3) and extracted with CH2Cl2. The organic layer was dried over MgSO4 and concentrated in vacuo to give 2-amino-3-methoxybenzenethiol (36.3 g). To a mixture of 2-amino-3-methoxybenzenethiol (36.3 g) in TFA (50 mL) was added trimethylsilyl polyphosphate (50 mL). After stirring for 2 h at 95 °C, the reaction mixture was concentrated in vacuo; water was added, and the resulting mixture was extracted with EtOAc. The organic layer was washed with satd NaHCO3 aq, water followed by brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (n-hexane-EtOAc = 30:1) to give 40 (44.7 g, 192 mmol, 69% over 2 steps) as a white solid. 1H NMR (CDCl3, 400 MHz): δ 4.08 (3H, s), 6.92 (1H, d, J = 8.6 Hz), 7.44-7.76 (2H, m).
References:
Ochiai, Koji;Takita, Satoshi;Eiraku, Tomohiko;Kojima, Akihiko;Iwase, Kazuhiko;Kishi, Tetsuya;Fukuchi, Kazunori;Yasue, Tokutaro;Adams, David R.;Allcock, Robert W.;Jiang, Zhong;Kohno, Yasushi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 5,p. 1644 - 1658] Location in patent:experimental part
5464-79-9
186 suppliers
$19.00/250mg

354760-24-0
3 suppliers
inquiry