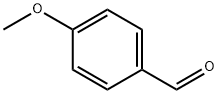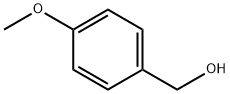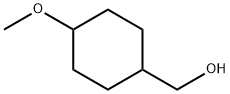
4-methoxyCyclohexanemethanol synthesis
- Product Name:4-methoxyCyclohexanemethanol
- CAS Number:101869-74-3
- Molecular formula:C8H16O2
- Molecular Weight:144.21
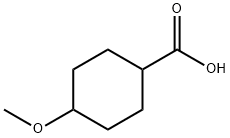
95233-12-8
65 suppliers
$30.00/250mg

101869-74-3
13 suppliers
inquiry
Yield:101869-74-3 91%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 4 h;Inert atmosphere;
Steps:
18.2 (4-Methoxycyclohexyl)methanol
Lithium aluminum hydride (92.8 mg, 2.45 mmol) was slowly added into the tetrahydrofuran (7 mL) containing methyl-4-methoxycyclohexanecarboxylic acid (280 mg, 1.63 mmol) at 0° C. under the protection of nitrogen.
The reaction solution was stirred at room temperature for 4 hours, and then cooled to 0° C. in an ice-water bath.
Water (0.1 mL), 15% sodium hydroxide (0.1 mL) and water (0.3 mL) was slowly added successively.
The reaction solution was heated to room temperature, and then stirred for half an hour, followed by filtration.
The filter cake was washed with tetrahydrofuran (8 mL*2) and the filtrate was concentrated under reduced pressure to give (4-methoxycyclohexyl)methanol (213 mg, yellow oily) with a yield of 91%. 1H NMR: (400 MHz, Methonal-d4) δ3.49-3.45 (m, 1H), 3.39-3.37 (m, 2H), 3.32 (s, 3H), 1.95-1.80 (m, 3H), 1.56-1.48 (m, 4H), 1.36-1.27 (m, 2H).
References:
US2018/148451,2018,A1 Location in patent:Paragraph 0195; 0198

223677-02-9
1 suppliers
inquiry

101869-74-3
13 suppliers
inquiry

137058-17-4
4 suppliers
inquiry

101869-74-3
13 suppliers
inquiry
3685-26-5
174 suppliers
$8.00/250mg

101869-74-3
13 suppliers
inquiry