(4-METHYL-1,3-THIAZOL-2-YL)ACETONITRILE synthesis
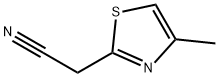
- Product Name:(4-METHYL-1,3-THIAZOL-2-YL)ACETONITRILE
- CAS Number:19785-39-8
- Molecular formula:C6H6N2S
- Molecular Weight:138.19
Yield: 54%
Reaction Conditions:
with triethylamine in DMF (N,N-dimethyl-formamide) at 40; for 1.5 h;
Steps:
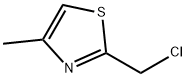
2 Description 2. [4-methylthiazol-2-yl]acetonitrile
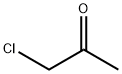
To a solution of a-cyanothioacetamide (2 g, 20 mmol) in DMF (10 mL) and triethylamine (2.8 mL, 20 mmol), [CHLOROACETONE] (1.6 mL, 20 mmol) was slowly added (dropping funnel). A sticky solid rapidly separated from the solution. The reaction mixture was heated at [40°C] for 1.5 hours. As judged by TLC [(SI02] ; hexane/AcOEt 6: 4, [RF=0.] 7) the reaction was complete. The suspension was poured onto water (110 mL) and extracted with AcOEt (3 x 150 mL). The combined organic phases were dried over [NA2SO4] and concentrated under vacuum. The oily residue was chromatographed on [A SIO2] column (eluent hexane/AcOEt gradient from 8: 2 to 1: 1) yielding pure [4-methylthiazol-2- yl] acetonitrile (2,1. 5 g, 54%) as a light-yellow oil. Analytical data ['H-NMR] [(DMSO-D6,] [8)] : 6.9 (s, 1H); 4.1 (s, 2H, CH2-CN) ; 2.4 (s, 3H)
References:
NOVUSPHARMA S.P.A. WO2003/105842, 2003, A1 Location in patent:Page 65
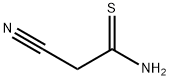
7357-70-2
175 suppliers
$15.00/1g
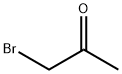
598-31-2
117 suppliers
$60.00/50mg

19785-39-8
64 suppliers
inquiry