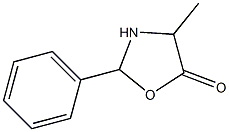
4-Methyl-2-phenyl-5-oxazolidinone synthesis
- Product Name:4-Methyl-2-phenyl-5-oxazolidinone
- CAS Number:70155-88-3
- Molecular formula:C10H11NO2
- Molecular Weight:177.1998
Yield:-
Reaction Conditions:
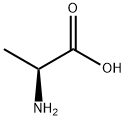
Stage #1: rac-Ala-OHwith sodium hydroxide in methanol;water;Heating;
Stage #2: benzaldehyde in ethanol at 20; for 3 h;
Steps:
3.1
(DL)-Alanine (32.9 g, 370 mmol) was added to a solution of NaOH (15.1 g, 370 mmol) in H2O (50 mL) followed by methanol (250 mL). The mixture was heated until the solid dissolved. The solvent was then evaporated until precipitation formed (30 mL residue). The residue was dissolved in ethanol (250 mL) and benzaldehyde (59 g, 556 mmol) was added. This mixture was stirred at room temperature for 3 h. Ethanol and most of water was removed under vacuum. The residue was dissolved in ethanol (200 mL) and dried over 4 molecular sieves. Filtration and evaporation of the solvent gave a white solid which was dried under vacuum overnight. This solid was suspended in dichloromethane (500 mL), and a solution of benzoyl chloride (52.0 g, 370 mmol) in dichloromethane (100 mL) was added dropwise at 0° C. After 3 h at 0° C., the reaction mixture was allowed to stir at room temperature overnight. This suspension/mixture was washed with H2O, 5% NaHCO3, 5% of NaHSO3, and H2O sequentially, then dried over Na2SO4. Evaporation of the solvent gave a white solid. Fractional recrystallizations of this solid from CH2Cl2 and ether (1:2) gave white crystals of 3-benzoyl-4-methyl-2-phenyloxazolidin-5-one (60 g, 58%). LC-MS (ES, m/z): 282 [M+H]+.
References:
US2011/275636,2011,A1 Location in patent:Page/Page column 26