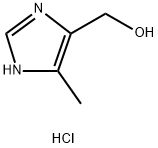
4-Methyl-5-imidazolemethanol hydrochloride synthesis
- Product Name:4-Methyl-5-imidazolemethanol hydrochloride
- CAS Number:38585-62-5
- Molecular formula:C5H9ClN2O
- Molecular Weight:148.59

60-29-7
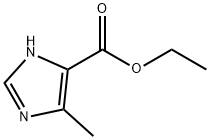
51605-32-4

38585-62-5
1. Liquid ammonia is collected in a reactor under well ventilated conditions. Sodium metal (335 g, 15.23 mol) was added in batches and stirred until completely dissolved, forming a dark blue solution. This process takes about 15 minutes. 2. ethyl 4-methyl-5-imidazolecarboxylate (500 g, 3.25 mol) was suspended in 400 mL of anhydrous ethanol to form a wet powder. Over a period of about 30 minutes, this wet powder was carefully added in batches to the sodium-ammonia solution described above. 3. Upon completion of the addition, methanol (1 L) was slowly added. Subsequently, ammonium chloride (810 g, 15.28 mol) was added cautiously until the blue color of the solution faded, at which time the addition of the remaining ammonium chloride could be accelerated. 4. After the ammonium chloride has been added, the ammonia is evaporated using a cold water bath. Gradually increase the temperature of the water bath as the volume of the reaction mixture decreases. When most of the ammonia has been removed, switch to steam heating and remove the residual ammonia completely under vacuum, a process that takes 7-15 hours. 5. Isopropanol (6 L) was added to the residue and refluxed under vigorous stirring for 1 hour. Water (100 mL) was then added and stirring was continued for 10 minutes. 6. The mixture was cooled to about 40 °C, passed through hydrogen chloride gas to acidity and subsequently filtered. The filter cake was washed with hot isopropanol, and the filtrate was combined and concentrated to about 1 L. The mixture was then purged with acetone (4 L). 7. The concentrate was diluted with acetone (4 L) and ether (2 L) and the precipitated solid product was collected. Finally, the product was dried under vacuum at 60 °C to give 4-methyl-5-hydroxymethylimidazole hydrochloride in 94% yield.
Yield:38585-62-5 94%
Reaction Conditions:
with ammonium chloride;sodium in methanol;ethanol;ammonia;water;isopropyl alcohol;acetone
Steps:
3 EXAMPLE 3
After the ammonia was collected, sodium (335 g., 15.23 m.) was added in portions and dissolved in the ammonia giving a deep blue solution. The addition of sodium required about 15 minutes. 5-Methyl-4-imidazolecarboxylic acid ethyl ester (500 g., 3.25 m.) was added to 400 ml. of dry ethanol giving a wet powder. This wet powder was added portionwise with caution to the sodium-ammonia solution over a period of about 30 minutes. After the addition was completed, one liter of methanol was added carefully. Ammonium chloride (810 g., 15.28 m.) was added very cautiously until the blue color was discharged whereupon the remaining ammonium chloride could be added more rapidly. After the addition of the ammonium chloride, the ammonia was evaporated using a cold water heating bath. As the volume of the mixture decreased, the heating bath was made warmer. When nearly all the ammonia was gone, the mixture was heated with steam under a vacuum to remove the last traces of ammonia. The removal of ammonia requires 7-15 hours. Isopropanol (6 liters) was added to the residue and refluxed for one hour with vigorous stirring. Water (100 ml.) was then added and the stirring continued for 10 minutes. The mixture was then cooled to about 40° C. and acidified with hydrogen chloride gas and filtered. The filter cake was washed with hot isopropanol and the combined filtrate concentrated to about one liter and diluted with 4 liters of acetone and 2 liters of ethyl ether. The product was collected and dried at 60° C. under vacuum to give 4-(hydroxymethyl)-5-methylimidazole hydrochloride, yield 94 percent.
References:
SK&F Lab Co. US4063023, 1977, A

51605-32-4
162 suppliers
$6.00/1g

38585-62-5
229 suppliers
$6.00/5g