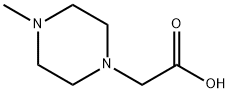
(4-METHYL-PIPERAZIN-1-YL)-ACETIC ACID synthesis
- Product Name:(4-METHYL-PIPERAZIN-1-YL)-ACETIC ACID
- CAS Number:54699-92-2
- Molecular formula:C7H14N2O2
- Molecular Weight:158.2
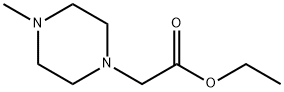
28920-67-4

54699-92-2
b) Synthesis of 4-methyl-1-piperazinylacetic acid Ethyl (4-methyl-1-piperazinyl) acetate (1.3 g, 6.50 mmol, 1.0 eq.) was dissolved in 8N hydrochloric acid and the reaction was stirred at 95°C for 16 hours. After completion of the reaction, the reaction solution was concentrated by vacuum rotary evaporator. The remaining hydrochloric acid was neutralized with sodium bicarbonate solution and subsequently extracted with ethyl acetate (3 x 10 ml). The organic phases were combined, washed sequentially with water and saturated saline and dried over anhydrous sodium sulfate. The organic solvent was removed by distillation under reduced pressure to afford the target product 4-methyl-1-piperazineacetic acid in 54.5% yield (0.6 g).LC-MS (ESI) analysis: theoretical mass: 158.0; measured mass: 159.1 [M + H]+ (retention time: 0.102 min).

60-29-7
0 suppliers
$30.00/50g

54699-92-2
133 suppliers
$8.00/250mg
Yield: 90%
Reaction Conditions:
in 1-acetoxy-2-(4-methyl-piperazino)-ethane;water
Steps:
3 Example 3
Example 3 Preparation of (4-methylpiperazinyl) acetic acid (VII) A 22 g solution of (4-methylpiperaziny) ethyl acetate (VI) in 500 ml of water was stirred at the reflux temperature of the solvent for 2 h. The reaction mixture was evaporated to dryness, thus obtaining a residue which, by grinding with a 8:2 diethylether/acetone mixture, provided 16.75 g (yield 90%) of a white solid with a melting point of 160-2 °C, identified as (4-methylpiperazinyl) acetic acid (VII).
References:
LABORATORIOS RUBIO, S.A. EP693481, 1996, A1

28920-67-4
37 suppliers
$55.00/250mg

54699-92-2
133 suppliers
$8.00/250mg
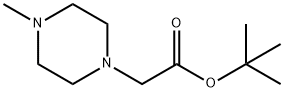
835916-40-0
0 suppliers
inquiry

54699-92-2
133 suppliers
$8.00/250mg
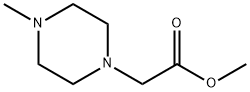
5780-70-1
42 suppliers
$151.58/1g

54699-92-2
133 suppliers
$8.00/250mg
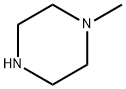
109-01-3
672 suppliers
$5.00/5 g
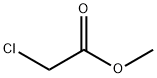
96-34-4
363 suppliers
$10.00/25g

54699-92-2
133 suppliers
$8.00/250mg