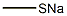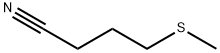
4-(Methylthio)butanenitrile synthesis
- Product Name:4-(Methylthio)butanenitrile
- CAS Number:59121-24-3
- Molecular formula:C5H9NS
- Molecular Weight:115.2
Yield: 100%
Reaction Conditions:
in ethanol at 20; for 25 h;Inert atmosphere;Cooling with ice;
Steps:
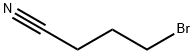
1 EXAMPLE 1 Synthesis of 4-methylthiobutyronitrile
EXAMPLE 1 Synthesis of 4-methylthiobutyronitrile [0074] 53.38 g of 4-chlorobutyronitrile (1 equivalent) in solution in 250 mL of ethanol are placed in an isobaric dropping funnel, surmounting a 1 liter flask. [0075] 1.1 equivalents of sodium methanethiolate, as an aqueous 21% sodium methanethiolate solution (Sigma-Aldrich) are introduced into the reactor. [0076] The flask is cooled by means of a water-ice bath and the system is degassed, and then placed under nitrogen and maintained with stirring. [0077] The solution containing the nitrile is then poured into the reactor within about one hour (rapidly dropwise). [0078] This time having elapsed, the ice bath is removed so as to return to room temperature. The stirring is then maintained for further 24 hours at this temperature. [0079] At the end of the reaction, 400 mL of water are added, as well as 200 mL of dichloromethane. The solution is decanted and the aqueous phase is extracted twice again with dichloromethane. The collected organic phases are then washed with water and then dried on magnesium sulfate and filtered. [0080] The solvent is finally removed in the rotary evaporator. 58.59 g of a colorless liquid are then recovered and engaged into the following step without requiring any additional purification. The yield is considered as quantitative. [0081] TLC: Eluent: Hexane-Et2O 50/50; [0082] Developer: phosphomolybdic acid; [0083] Rf=0.45 [0084] 1H NMR (300 MHz; CDCl3): [0085] δ (ppm): 2.62 (t, 2H, J=6.6 Hz, CH2CN); 2.51 (t, 2H, J=7.0 Hz, CH2SCH3); 2.10 (s, 3H, CH2SCH3); 1.94 (m, 2H, CH2CH2CH2) [0086] 13C NMR (75 MHz; CDCl3): [0087] δ (ppm): 119.1; 32.6; 24.6; 15.8; 15.3 [0088] Refractive index: nD20=1.4814
References:
Auriga International;Dubois, Jacques;Marchal, Alfred;Lacroix, Damien;Cabou, Jérôme US2013/142739, 2013, A1 Location in patent:Paragraph 0074-0088