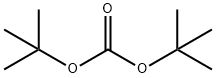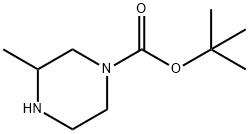
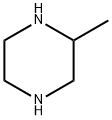
4-N-Boc-2-Methyl-piperazine synthesis
- Product Name:4-N-Boc-2-Methyl-piperazine
- CAS Number:120737-59-9
- Molecular formula:C10H20N2O2
- Molecular Weight:200.28

24424-99-5
109-07-9

120737-59-9
General procedure for the synthesis of 4-tert-butoxycarbonyl-2-methylpiperazine from di-tert-butyl dicarbonate and 2-methylpiperazine: 1) Di-tert-butyl dicarbonate (21.7 g) was slowly added to a solution of 2-methylpiperazine (10.0 g) in methanol (200 mL) at room temperature, followed by continuous stirring of the reaction mixture for 24 hours. Upon completion of the reaction, the methanol solvent was removed by distillation under reduced pressure. The resulting crude product was purified by silica gel column chromatography (eluent: chloroform-methanol mixed solvent) to finally obtain tert-butyl 3-methylpiperazine-1-carboxylate as a colorless oily product (19.3 g, 96.5% yield). The product was characterized by 1H-NMR (300 MHz, CDCl3): δ1.04 (3H, d, J=6.24 Hz), 1.46 (9H, s), 2.39 (1H, br s), 2.70-2.77 (3H, m), 2.94 (1H, br s), 3.93 (2H, br s). Mass spectrometry analysis (FAB) showed the molecular ion peak M/Z: 201 (M+H)+.

24424-99-5
871 suppliers
$13.50/25G

109-07-9
387 suppliers
$5.00/5g

120737-59-9
172 suppliers
$6.00/1g
Yield:120737-59-9 100%
Reaction Conditions:
in dichloromethane;
Steps:
192.1 Tert-Butyl 4-(6-ethylthiopheno[3,2-e]pyrimidin-4-yl)-3-methylpiperazinecarboxylate
Step 1: 2-Methylpiperazine (5.03 g) was dissolved in dichloromethane (200 mL) and a solution of di-tert-butyl-dicarbonate (10.96 g) in dichloromethane (100 mL) was added over 2.5 hours. The reaction was stirred at room temperature for 24 hours and concentrated to dryness giving 1-BOC-3-methylpiperazine. Yield=100%
References:
US2003/153556,2003,A1
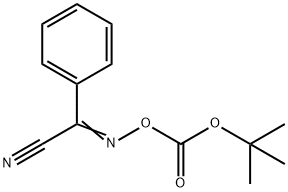
58632-95-4
271 suppliers
$7.00/5g

109-07-9
387 suppliers
$5.00/5g

120737-59-9
172 suppliers
$6.00/1g
![Benzeneacetonitrile, α-[(2,2-dimethyl-1-oxopropoxy)imino]-](/CAS/20210305/GIF/91612-43-0.gif)