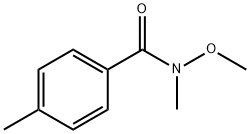
4,N-DIMETHYL-N-METHOXYBENZAMIDE synthesis
- Product Name:4,N-DIMETHYL-N-METHOXYBENZAMIDE
- CAS Number:122334-36-5
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
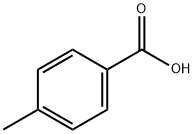
99-94-5
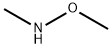
1117-97-1

122334-36-5
GENERAL PROCEDURE: Methoxymethylamine (0.360 g, 6.0 mmol) and p-methylbenzoic acid (0.244 g, 2.0 mmol) were dissolved in anhydrous toluene (10 mL) and stirred for 10 min at 0 °C. Subsequently, a solution of phosphorus trichloride (0.137 g, 1.0 mmol) in anhydrous toluene (2 mL) was slowly added dropwise to the above mixture. The reaction mixture was gradually warmed up to room temperature, followed by stirring at 60 °C for 0.5 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the mixture was cooled to room temperature. The reaction was quenched with saturated sodium bicarbonate solution (20 mL) and extracted with ethyl acetate (3 x 10 mL). The organic layers were combined and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure and the resulting crude product was purified by column chromatography (silica gel, petroleum ether-ethyl acetate, 3:2) to afford the colorless oily target product N-methoxy-N,4-dimethylbenzamide (320 mg, 97% yield).
Yield: 98%
Reaction Conditions:
Stage #1:p-Toluic acid;N,0-dimethylhydroxylamine in toluene at 0; for 0.166667 h;
Stage #2: with phosphorus trichloride in toluene at 20 - 60; for 0.5 h;
Steps:
N-Methoxy-N-methylbenzamide (3a);38,39 Typical Procedure
General procedure: A solution of NHMe(OMe) (0.360 g, 6.0 mmol) and benzoic acid (0.244 g, 2.0 mmol) was stirred in dry toluene (10 mL) at 0 °C for 10 min. A solution of PCl3 (0.137 g, 1.0 mmol) in dry toluene (2 mL) was then added dropwise to the mixture. The mixture was warmed to r.t. slowly and then stirred at 60 °C for 0.5 h. When the reaction was complete (TLC monitoring), the mixture was cooled to r.t. The mixture was then quenched with sat. NaHCO3 soln (20 mL) and extracted with EtOAc (3 × 10 mL). The combined organic layers were dried (anhyd MgSO4). The solvent was removed in vacuo.The product was purified by column chromatography (silica gel, petroleum ether-EtOAc, 3:2) to give pure 3a as a colorless oil; yield: 320 mg (97%).
References:
Niu, Teng;Wang, Ke-Hu;Huang, Danfeng;Xu, Changming;Su, Yingpeng;Hu, Yulai;Fu, Ying [Synthesis,2014,vol. 46,# 3,art. no. SS-2013-H0629-OP,p. 320 - 330]
589-18-4
316 suppliers
$5.00/5g

6638-79-5
595 suppliers
$6.00/25g

122334-36-5
65 suppliers
$18.00/100mg

6638-79-5
595 suppliers
$6.00/25g

99-94-5
614 suppliers
$9.00/1g

122334-36-5
65 suppliers
$18.00/100mg

6638-79-5
595 suppliers
$6.00/25g
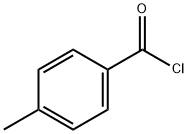
874-60-2
416 suppliers
$10.00/5g

122334-36-5
65 suppliers
$18.00/100mg
201230-82-2
1 suppliers
inquiry
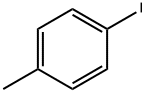
624-31-7
439 suppliers
$9.00/25g

6638-79-5
595 suppliers
$6.00/25g

122334-36-5
65 suppliers
$18.00/100mg