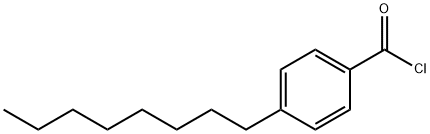
4-OCTYLBENZOYL CHLORIDE synthesis
- Product Name:4-OCTYLBENZOYL CHLORIDE
- CAS Number:50606-97-8
- Molecular formula:C15H21ClO
- Molecular Weight:252.78
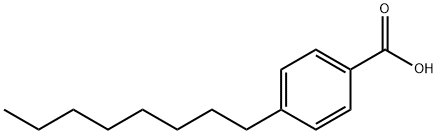
3575-31-3
117 suppliers
$48.00/1g

50606-97-8
25 suppliers
$420.61/1gm:
Yield: 100%
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 2 h;
Steps:
G
An acid (0.28 mmols) and a catalytic amount of dimethylformamide were dissolved in dichloromethane (2.8 mLs) and the solution was cooled to 0° C. To this solution was added oxalyl chloride (0.84 mmols) and the reaction mixture was warmed to room temperature. After stirring for 2 hours, the reaction mixture was evaporated to dryness; the crude material was taken up in 3 one mL portions of diethyl ether and evaporated. The oil was dried under vacuum for an additional 30 minutes and carried on. General procedure G was used to convert 0.53 mmols of 4-octylbenzoic acid to an acid chloride. After standard purification techniques, the acyl-chloride was isolated in quantitative yield and carried on immediately. General procedure F was used to couple 0.41 mmols of 1-amino-1-cyclopropanecarbonitrile to 0.53 mmols of the acid chloride. Standard purification techniques were used to isolate 0.21 mmols (51%) of the title product. 1H NMR (500 MHz, CDCl3) δ 7.74-7.67 (m, 2H), 7.21 (d, J=8.4, 2H), 7.12 (s, 1H), 2.68-2.58 (m, 2H), 1.59 (dd, J=5.8, 8.3, 4H), 1.35 (dd, J=5.9, 8.3, 2H), 1.33-1.21 (m, 10H), 0.87 (t, J=7.0, 3H). 13C NMR (126 MHz, CDCl3) δ 168.06, 148.02, 129.96, 128.73, 127.27, 120.23, 35.85, 31.83, 31.13, 29.39, 29.21, 22.63, 20.88, 16.91, 14.08.
References:
University of Virginia Patent Foundation US2012/214858, 2012, A1 Location in patent:Page/Page column 19; 21

79-37-8
490 suppliers
$17.67/10gm:
2189-60-8
222 suppliers
$30.00/20mg

50606-97-8
25 suppliers
$420.61/1gm:
619-58-9
450 suppliers
$8.00/5g

50606-97-8
25 suppliers
$420.61/1gm:
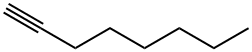
629-05-0
169 suppliers
$10.00/1g

50606-97-8
25 suppliers
$420.61/1gm:
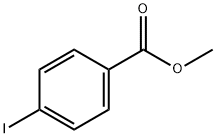
619-44-3
318 suppliers
$6.00/10g

50606-97-8
25 suppliers
$420.61/1gm: