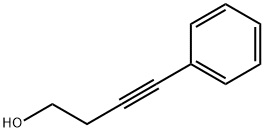
4-PHENYL-3-BUTYN-1-OL synthesis
- Product Name:4-PHENYL-3-BUTYN-1-OL
- CAS Number:10229-11-5
- Molecular formula:C10H10O
- Molecular Weight:146.19
Yield: 100%
Reaction Conditions:
with copper(l) iodide;tetrakis(triphenylphosphine) palladium(0);triethylamine for 16 h;Reflux;Inert atmosphere;Schlenk technique;Sonogashira Cross-Coupling;
Steps:
4-Phenylbut-3-yn-1-ol (17a)
Iodobenzene (16a, 0.20 mL, 1.83 mmol, 1 eq) was dissolved under N2 atmosphere in triethylamine (20 mL). CuI (7.0 mg, 0.04 mmol, 0.02 eq), Pd(PPh3)4 (63.4 mg, 0.05 mmol, 0.03 eq) and but-3-yn-1-ol (7, 0.20 mL, 2.71 mmol, 1.48 eq.) were added and the solution was heated to reflux for 16 h. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography (d = 2 cm, h = 15 cm, V = 10 mL, cyclohexane:ethyl acetate = 2:1 ). Orange oil, yield 267 mg (1.83 mmol, 100 %). C10H10O (146.2). Rf = 0.35 (cyclohexane:ethyl acetate = 2:1). HPLC: 97.5 %, tR = 17.18 min. 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.79 - 1.95 (m, 1H, OH), 2.70 (t, J = 6.3 Hz, 2H, PhCCCH2CH2OH), 3.82 (t, J = 6.3 Hz, 2H, PhCCCH2CH2OH), 7.27 - 7.45 (m, 5H, phenyl). 13C NMR (101 MHz, CDCl3): δ [ppm] = 24.0 (1C, PhCCCH2CH2OH), 61.3 (1C, PhCCCH2CH2OH), 82.6 (1C, PhCCCH2CH2OH), 86.5 (1C, PhCCCH2CH2OH), 123.5 (1C, C-1phenyl), 128.1, 128.4, 131.8 (5C, C-2phenyl, C-3phenyl, C-4phenyl, C-5phenyl and C-6phenyl). IR: v[cm-1] = 3364 (O-H), 3032 (=C-H), 2932, 2886 (C-Haliph.), 1678, 1597, 1489 (C=Carom.), 1443 (CH2 deform.), 1042 (C-OH). Exact Mass (APCI): m/z = 147.0804 (calcd. 147.0806 for C10H11O [MH]+).
References:
Wagner, Marina;Schepmann, Dirk;Ametamey, Simon M.;Wünsch, Bernhard [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 16,p. 3559 - 3567] Location in patent:supporting information
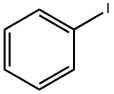
591-50-4
500 suppliers
$10.00/1g
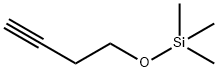
17869-75-9
33 suppliers
$102.55/10 G

10229-11-5
21 suppliers
inquiry
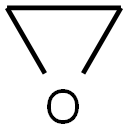
75-21-8
239 suppliers
$33.29/crm44609
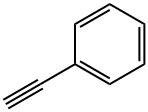
536-74-3
315 suppliers
$10.00/1g

10229-11-5
21 suppliers
inquiry

75-21-8
239 suppliers
$33.29/crm44609
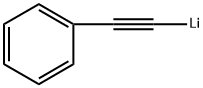
4440-01-1
18 suppliers
$45.00/1ml

10229-11-5
21 suppliers
inquiry

