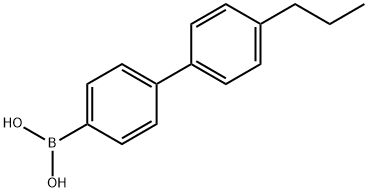
(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid synthesis
- Product Name:(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid
- CAS Number:153035-56-4
- Molecular formula:C15H17BO2
- Molecular Weight:240.11
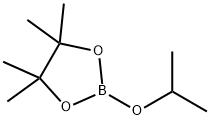
61676-62-8
324 suppliers
$10.00/5g
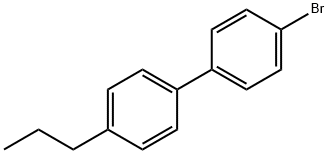
58743-81-0
152 suppliers
$9.00/5g
![(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid](/CAS2/GIF/153035-56-4.gif)
153035-56-4
107 suppliers
$13.00/1g
Yield: 80%
Reaction Conditions:
Stage #1:4-n-propyl-4'-bromobiphenyl in tetrahydrofuran at -78; for 1 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran at 20; for 1 h;
Steps:
2.1
[Experimental embodiment 2]: preparation of the compound in formula (I-4)Step 1: synthesize the compound in formula (2-A) (hereinafter “compound (2-A)”).First, 10 grams of 4-bromo-1-propyl benzene in formula (2) is placed in a two-neck bottle. The two-neck bottle is heated, and waterless THF is injected into the two-neck bottle. After the two-neck bottle is cooled to -78° C., 18 mL of an n-BuLi solution is slowly dripped into the two-neck bottle. After maintaining the reaction temperature at -78° C. for 1 hour, 10 mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane is slowly dripped into the two-neck bottle. After continuing the reaction for 1 hour, the bottle is heated to room temperature. 10 mL of distilled water is added to the above double-neck bottle, and low pressure evaporation-concentration is performed to remove the THF. Ethyl acetate and distilled water are used to extract the obtained mixture solution. Afterwards, the collected organic solution is desiccated by waterless magnesium sulfate, and the solid magnesium sulfate is removed by gravitational filtration. Then, low pressure evaporation-concentration is performed on the obtained mixture solution to remove the solvent. 9.47 grams of a white solid compound (2-A) is obtained. The yield is 80%.
References:
DAXIN MATERIALS CORPORATION US2012/264984, 2012, A1 Location in patent:Page/Page column 4-5
688-71-1
133 suppliers
$45.00/25mL

58743-81-0
152 suppliers
$9.00/5g
![(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid](/CAS2/GIF/153035-56-4.gif)
153035-56-4
107 suppliers
$13.00/1g
92-66-0
582 suppliers
$10.00/1g
![(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid](/CAS2/GIF/153035-56-4.gif)
153035-56-4
107 suppliers
$13.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid](/CAS2/GIF/153035-56-4.gif)
153035-56-4
107 suppliers
$13.00/1g

58743-81-0
152 suppliers
$9.00/5g
![(4'-Propyl[1,1'-biphenyl]-4-yl)-boronic acid](/CAS2/GIF/153035-56-4.gif)
153035-56-4
107 suppliers
$13.00/1g