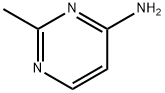
4-Pyrimidinamine, 2-methyl- (9CI) synthesis
- Product Name:4-Pyrimidinamine, 2-methyl- (9CI)
- CAS Number:74-69-1
- Molecular formula:C5H7N3
- Molecular Weight:109.13
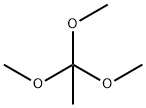
1445-45-0

60838-50-8

74-69-1
The general procedure for the synthesis of 2-methyl-4-aminopyrimidine from trimethyl orthoacetate and 3-methoxyacrylonitrile was as follows: in a 10 mL stainless steel pressure-resistant reactor, 1.0 g (12 mmol) of 3-methoxyacrylonitrile, 3.9 g (32 mmol) of trimethyl orthoacetate, and 4.0 g (56 mmol) of 24 wt% ammonia-methanol solution were added in sequence. The reaction mixture was stirred at 130°C for 8 hours. After completion of the reaction, 20 mL of hexane was added to the reaction mixture, stirred thoroughly and filtered to afford 0.94 g (isolated yield: 72%) of the target product 2-methyl-4-aminopyrimidine as yellow crystals. 2-methyl-4-aminopyrimidine was characterized physically as follows: 1H-NMR (DMSO-d6, δ/ppm): 2.29 (3H, s), 6.22 (1H, dd, J = 5.9), 4.0 g (56 mmol) of 24 wt% ammonia-methanol solution. dd, J = 5.9, 0.5 Hz), 6.69 (2H, brs), 7.94 (1H, d, J = 5.9 Hz); CI-MS (m/e): 109 (M + 1).

1445-45-0
359 suppliers
$10.00/5mL

60838-50-8
88 suppliers
$60.00/100mg

74-69-1
119 suppliers
$19.00/100mg
Yield: 72%
Reaction Conditions:
with ammonia in methanol at 130; for 8 h;
Steps:
4
Example 4; Synthesis of 2-methyl-4-aminopyrimidine; In a pressure-resistant vessel made of stainless steel having an inner volume of 10 ml were charged 1.0 g (12 mmol) of 3-methoxyacrylonitrile, 3.9 g (32 mmol) of methyl orthoacetate and 4.0 g (56 mmol) of 24% by weight ammonia-methanol solution, and the mixture was reacted under stirring at 130° C. for 8 hours. After completion of the reaction, 20 ml of hexane was added to the reaction mixture, and the mixture was stirred and then filtered to obtain 0.94 g (Isolation yield: 72%) of 2-methyl-4-aminopyrimidine as yellow crystals. Physical properties of the 2-methyl-4-aminopyrimidine were as follows. 1H-NMR (DMSO-d6, δ (ppm)); 2.29 (3H, s), 6.22 (1H, dd, J=5.9, 0.5 Hz), 6.69 (2H, brs), 7.94 (1H, d, J=5.9 Hz) CI-MS (m/e); 109 (M+1)
References:
Nishino, Shigeyoshi;Hirotsu, Kenji;Shima, Hidetaka;Harada, Takashi US2008/45712, 2008, A1 Location in patent:Page/Page column 3-4
1749-68-4
143 suppliers
$8.00/1g

74-69-1
119 suppliers
$19.00/100mg
4994-86-9
209 suppliers
$16.00/1g

74-69-1
119 suppliers
$19.00/100mg
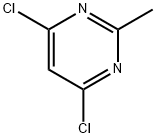
1780-26-3
477 suppliers
$10.00/10g

74-69-1
119 suppliers
$19.00/100mg
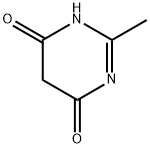
40497-30-1
346 suppliers
$14.00/5g

74-69-1
119 suppliers
$19.00/100mg