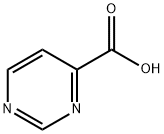
4-Pyrimidinecarboxylic acid synthesis
- Product Name:4-Pyrimidinecarboxylic acid
- CAS Number:31462-59-6
- Molecular formula:C5H4N2O2
- Molecular Weight:124.1
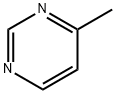
3438-46-8

31462-59-6
1. Oxidation reaction: Selenium dioxide (8.82 g, 0.079 mol) was slowly added to a stirred solution of 4-methylpyrimidine (5.0 g, 0.053 mol) in pyridine (55 mL) at room temperature. The reaction mixture was stirred at 55°C for 2 hours, then warmed to 80°C and continued stirring for 3.5 hours. After completion of the reaction, it was cooled to room temperature and stirred overnight, the reaction mixture was filtered and the residue was washed with pyridine. The pyridine solution was combined and concentrated, and the resulting pyrimidine-4-carboxylic acid was washed with water to remove traces of selenium dioxide. Yield: 5.3 g, 80.5% yield. 2. Esterification reaction: Concentrated pyrimidine-4-carboxylic acid (5.0 g, 0.04 mol) was dissolved in methanol (170 mL) and hydrochloric acid (2 mL, room temperature) was added. After refluxing overnight, the reaction mixture was cooled to room temperature, neutralized with 10% sodium bicarbonate solution and concentrated. The esterified product was extracted with ether, dried over anhydrous sodium sulfate and concentrated to give methyl pyrimidine-4-carboxylate as a yellow solid. Yield: 3.3 g, yield 57.55%. 3. Amidation reaction: Trimethylacetyl chloride (11.30g, 0.093mol) was added dropwise to a solution of triethylamine (15.75g, 0.155mol) and 4-chloroaniline (10.0g, 0.078mol) in benzene (500mL) at 0℃. The reaction mixture was gradually warmed to room temperature and stirred for 3 hours. Upon completion of the reaction, it was quenched with water, extracted with ethyl acetate, washed sequentially with water and brine, and dried over anhydrous sodium sulfate. The resulting solid product was recrystallized from petroleum ether. Yield: 14.0 g, 84.43% yield. 4. Lithiation reaction: To a solution of N-(4-chlorophenyl)-2,2-dimethylpropanamide (3.5 g, 0.0165 mol) in tetrahydrofuran (50 mL) was added a hexane solution of n-butyllithium (2.64 g, 1.2 M, 0.041 mol) at 0 °C. The reaction was followed by stirring for 2 hours. After stirring at 0 °C for 2 h, the reaction was cooled to -70 °C and a tetrahydrofuran (25 mL) solution of methyl pyrimidine-4-carboxylate (3.18 g, 0.023 mol) was slowly added. The reaction mixture was then warmed to room temperature and stirred overnight. Ether (50 mL) and water (50 mL) were added and the organic layer was separated. The aqueous layer was further extracted with ether and the organic layers were combined, washed sequentially with water and brine and dried over anhydrous sodium sulfate. After concentration, the product was purified by column chromatography. Yield: 1.7 g, yield 32.69%. 5. Deprotection reaction: 70% sulfuric acid solution (10 mL) of protected aminoketone (1.7 g, 0.0054 mol) was heated at 95 °C overnight. After cooling to room temperature, it was alkalized with 10% sodium hydroxide solution, extracted with dichloromethane, washed sequentially with water and brine, and dried over anhydrous sodium sulfate. After concentration, the product was purified by basic alumina column chromatography to obtain the target compound. Yield: 0.20 g, 16% yield.

3438-46-8
226 suppliers
$9.00/5g

31462-59-6
224 suppliers
$8.00/1g
Yield:31462-59-6 80.5%
Reaction Conditions:
with selenium(IV) oxide in pyridine at 20 - 80; for 5.5 h;
Steps:
20 Example 20: Synthesis of (2-Amino-5-chloro-phenyl)-pyrimidin-4-yl-methanone
To 4-Methyl pyrimidine (5.0 g, 0.053 mol) in pyridine (55ML) was added selenium dioxide (8.82 g, 0.079 mol) at RT with stirring. The reaction mixture was stirred at 55 °C for 2 h and at 80 °C for 3.5 hr. After cooling to RT and stirring over night, the reaction mixture was filtered and the residue was washed with pyridine. The combined pyridine solution was concentrated and the carboxylic acid obtained was washed with water to remove traces of selenium dioxide. Yield : 5.3 g, 80.5 %. [00157] To Pyrimidine-4-carboxylic acid (5.0 g, 0.04 mol) in methanol (170 ml) was added conc. HCI (2 ML) at RT. After refluxing overnight, the reaction mixture was cooled to RT and neutralized with 10 % sodium bicarbonate solution and concentrated. The ester was extracted with diethyl ether, dried over NA2SO4 AND concentrated to get the methyl ester as a yellow solid, yield : 3.3 g, 57.55 %. [00158] TRIMETHYL ACETYLCHLORIDE (11.30 g, 0.093 mol) was added to a benzene (500 ML) solution of triethylamine (15.75 g, 0.155 mol) and 4- CHLOROANILINE (10.0 g, 0.078 mol) at 0°C. The reaction mixture was warmed to RT and stirred for 3h. The reaction mixture was then quenched with water, extracted with ethyl acetate, washed with water, brine solution and dried over NA2SO4. The solid product obtained was crystallized from pet ether. Yield : 14.0 g, 84. 43 %. [00159] To N- (4-chlorophenyl)-2, 2-dimethyl propanamide (3.5 g, 0.0165 mol) in THF (50 MI) at 0 °C was added n-butyl lithium in hexane (2.64 g, 1.2 M, 0. 041 MOL). Stirring was continued at 0 °C for 2 h, the reaction then cooled to-70 °C, pyrimidine-4-methyl carboxylate (3.18 g, 0.023 mol) in THF (25 ML) was then added slowly and the solution was warmed to RT and stirred overnight. Diethyl ether (50 ml) and water (50 ml) were added and the organic layer was separated. The aqueous layer was further extracted with ether. The combined ether layers were washed with water, brine and dried over NA2SO4. The product obtained upon concentration was purified by column chromatography. Yield : 1.7 g, 32.69 %. [00160] The protected amino ketone (1.7 g, 0.0054 mol) in sulfuric acid (10 ML, 70 %) was heated at 95 °C over night. The reaction mixture was cooled to RT and basified with 10% NAOH, extracted with DCM, washed with water, brine and dried over NA2SO4. The product obtained upon concentration was purified by column chromatography using basic alumina to yield title compound (0.20g, 16%).
References:
WO2004/46092,2004,A2 Location in patent:Page 52-53
![[1,2,3]Triazolo[1,5-c]pyrimidine](/CAS/20210111/GIF/49689-16-9.gif)
49689-16-9
0 suppliers
inquiry

31462-59-6
224 suppliers
$8.00/1g
2435-50-9
128 suppliers
$36.00/100mg

3438-46-8
226 suppliers
$9.00/5g

31462-59-6
224 suppliers
$8.00/1g

2435-50-9
128 suppliers
$36.00/100mg

3438-46-8
226 suppliers
$9.00/5g

31462-59-6
224 suppliers
$8.00/1g
1029598-59-1
0 suppliers
inquiry
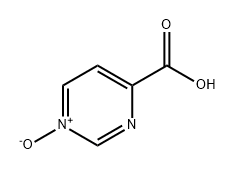
1029598-57-9
0 suppliers
inquiry

3438-46-8
226 suppliers
$9.00/5g
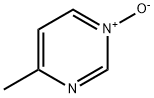
17758-54-2
3 suppliers
inquiry
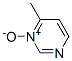
33342-83-5
4 suppliers
inquiry

31462-59-6
224 suppliers
$8.00/1g

1029598-59-1
0 suppliers
inquiry

1029598-57-9
0 suppliers
inquiry