FERROCENEACETIC ACID synthesis
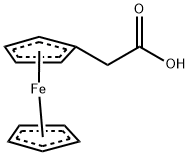
- Product Name:FERROCENEACETIC ACID
- CAS Number:1287-16-7
- Molecular formula:C12H12FeO2
- Molecular Weight:244.07
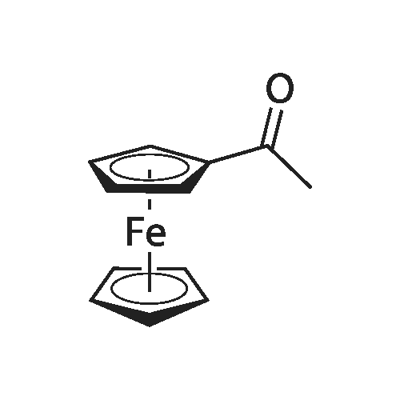
1271-55-2

1287-16-7
The general procedure for the synthesis of ferrocene acetic acid from acetylferrocene was as follows: 0.01 mol of acetylferrocene, 0.1 mol of morpholine, 0.05 mol of sulfur powder, and 0.001 mol of Na2S-9H2O were sequentially added to a 500 mL three-necked flask equipped with a mechanical stirrer and a spherical condenser, and the system was heated in an oil bath to 128 °C, and the reaction was maintained at reflux for 7 h, during which the reaction was monitored by thin-layer chromatography (TLC). for 7 h, during which the reaction progress was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the mixture was cooled to room temperature, followed by the addition of 10 mL of ethanol and 25 mL of 2 mol/L aqueous sodium hydroxide solution, stirred and heated to reflux for 4 h. The reaction continued to be monitored by TLC. After the reaction was complete, the reaction mixture was cooled, the pH was adjusted to 7-8 with dilute hydrochloric acid, and the precipitate was filtered and washed. Subsequently, the pH of the filtrate was adjusted to 1 with hydrochloric acid, left overnight, the precipitate was collected by filtration and washed to give the yellow-brown solid product ferrocene acetic acid in 79% yield.

1271-55-2
225 suppliers
$8.00/1g

1287-16-7
114 suppliers
$22.39/500mg
Yield:1287-16-7 79%
Reaction Conditions:
Stage #1:acetylferrocene with morpholine;sodiumsulfide nonahydrate;sulfur at 128; for 7 h;
Stage #2: with sodium hydroxide in ethanol;water for 4 h;Reflux;Temperature;
Steps:
1.1 Preparation of ferrocenyl acetic acid:
To a 500 mL three-necked flask equipped with a mechanical stirrer and a spherical condenser were added 0.01 mol of acetyl ferrocene,0.1 mol morpholine,0.05 mol of sulfur and 0.001 mol of Na2S · 9H2O, Oil bath was heated to 128 deg C, Reflux 7h, TLC monitoring.The reaction is completed,The mixture was cooled to room temperature,Then, 10 mL of ethanol and 25 mL of 2 mol / L sodium hydroxide aqueous solution were added thereto,Stirring temperature,Reflux 4h,TLC monitoring.The reaction is completed,cool down,Adjust the pH value with hydrochloric acid to between 7 and 8,Filter cleaning,The filtrate was then adjusted to pH 1 with hydrochloric acid,Placed overnight,filter,Washed,A yellow-brown solid,Namely ferrocenyl acetic acid,The yield is 79%.
References:
Shaanxi University of Science and Technology;Liu, Yuting;Song, Simeng;Yin, Dawei;Jiang, Shanshan;Liu, Beibei;Yang, Aning;Wang, Jinyu;Lyu, Bo CN104231004, 2017, B Location in patent:Paragraph 0033; 0042; 0051; 0060; 0069