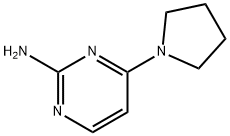
4-(pyrrolidin-1-yl)pyrimidin-2-amine synthesis
- Product Name:4-(pyrrolidin-1-yl)pyrimidin-2-amine
- CAS Number:1215986-09-6
- Molecular formula:C8H12N4
- Molecular Weight:164.21
Yield:1215986-09-6 89.7%
Reaction Conditions:
with potassium carbonate in 1-methyl-pyrrolidin-2-one at 20 - 120;Inert atmosphere;
Steps:
16.1 4-(Pyrrolidin-1-yl)pyrimidin-2-amine
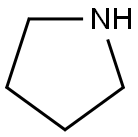
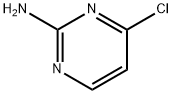
A light yellow suspension of 4-chloropyrimidin-2-amine (5.01 g, 38.7 mmol), pyrrolidine (2.50 g, 2.91 ml, 35.2 mmol) and potassium carbonate (9.72 g, 70.3 mmol) in N-methyl-2- pyrrolidinone (15.0 mL) was stirred under argon at 120 °C for 2 h and at room temperature overnight. The reaction mixture was cooled to room temperature and was poured into NaOH (1 M, 200 mL) and ice. The aqueous layer was extracted with ethyl acetate (200 mL) whereof part of the product precipitated in the water phase. The precipitate was filtered, washed water, triturated with dichloromethane and dried to obtain 3.1 g product as white solid. The separated ethyl acetate phase was dried over MgS04, filtered and concentrated in vacuum. The resulting light yellow solid was triturated with dichloromethane and dried to obtain 1.1 g product as white solid. The aqueous phase was extracted with dichloromethane (3 x 100 mL), dried over MgS04, filtered and concentrated in vacuum. The resulting light yellow solid was triturated with dichloromethane to obtain another 0.52 g product as white solid. The dichloromethane layers were combined, concentrated in vacuum and the product was purified by flash chromatography (using silica gel and a dichloromethane/methanol/ammonia gradient) to yield another 0.46 g product as light yellow. In total 5.18 g (89.7 ) of a light yellow solid were obtained. MS: m/z = 165.3 (M+H)+
References:
WO2014/187762,2014,A1 Location in patent:Page/Page column 42-43