
4-(TetraMethyl-1,3,2-dioxaborolan-2-yl)butan-2-one synthesis
- Product Name:4-(TetraMethyl-1,3,2-dioxaborolan-2-yl)butan-2-one
- CAS Number:100818-32-4
- Molecular formula:C10H19BO3
- Molecular Weight:198.07
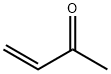
78-94-4
0 suppliers
$32.86/25ml

73183-34-3
586 suppliers
$6.00/5g

100818-32-4
14 suppliers
$45.00/50mg
Yield:100818-32-4 96%
Reaction Conditions:
with 2-((1,3-bis(N-butylimidazol-2-ylidene)phenylene)(dimethylamido)bis(iodo))rhodium(III);2-((1,3-bis(N-butylimidazol-2-ylidene)phenylene)(dimethylamido)bis(chloro))rhodium(III) in ethanol at 22; for 1 h;Catalytic behavior;Sealed tube;Solvent;
Steps:
4.5 General procedure for catalyses
General procedure: A 1 dram vial containing a magnetic stir bar was charged with α,β-unsaturated compound (0.0735mmol), bis(pinacolato)diboron (0.184mmol), pre-catalyst A (2.00mg, 2.94μmol), and solvent (0.700mL). The vial was closed and stirred vigorously for 1h at 22°C during which time a dark precipitate was observed to form. After 1h, a 10μL aliquot was passed through a short plug of celite and a 0.45 um PTFE filter. It was injected into the GC-MS to determine conversion. The crude reaction mixture was then filtered through a plug of celite. Volatiles were then removed under reduced pressure resulting in an oil. 1H NMR and HRMS was taken of the crude product to confirm the borylated product. The crude product was then purified by silica gel chromatography to determine an isolated yield.
References:
Reilly, Sean W.;Akurathi, Gopalakrishna;Box, Hannah K.;Valle, Henry U.;Hollis, T. Keith;Webster, Charles Edwin [Journal of Organometallic Chemistry,2016,vol. 802,p. 32 - 38]

7732-18-5
507 suppliers
$13.50/100ML

78-94-4
0 suppliers
$32.86/25ml

73183-34-3
586 suppliers
$6.00/5g

100818-32-4
14 suppliers
$45.00/50mg

7732-18-5
507 suppliers
$13.50/100ML

78-94-4
0 suppliers
$32.86/25ml
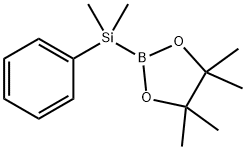
185990-03-8
88 suppliers
$17.00/250mg

100818-32-4
14 suppliers
$45.00/50mg
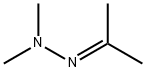
13483-31-3
43 suppliers
$110.00/2.5g
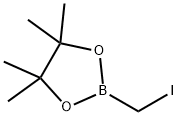
70557-99-2
78 suppliers
$6.00/1g
111-42-2
867 suppliers
$10.00/20mg

100818-32-4
14 suppliers
$45.00/50mg

70557-99-2
78 suppliers
$6.00/1g

100818-32-4
14 suppliers
$45.00/50mg