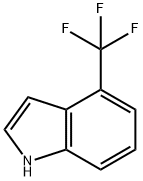
4-(TRIFLUOROMETHYL)-1H-INDOLE synthesis
- Product Name:4-(TRIFLUOROMETHYL)-1H-INDOLE
- CAS Number:128562-95-8
- Molecular formula:C9H6F3N
- Molecular Weight:185.15
![EthenaMine, N,N-diMethyl-2-[2-nitro-6-(trifluoroMethyl)phenyl]-, (1E)-](/CAS/20150408/GIF/905274-06-8.gif)
905274-06-8

128562-95-8
Step 2. Synthesis of 4-trifluoromethyl-1H-indole: Dimethyl-[2-(2-nitro-6-trifluoromethyl-phenyl)-vinyl]-amine (6.3 g, 24 mmol) was dissolved in acetic acid (150 mL) and iron powder (4.3 g, 77 mmol) was added. The reaction mixture was heated to reflux and kept overnight under nitrogen protection. After completion of the reaction, it was cooled to room temperature, the reaction mixture was diluted with 2.0 M hydrochloric acid and the aqueous phase was extracted with ethyl acetate. The organic layer was neutralized with saturated potassium carbonate solution and extracted again. Subsequently, the organic layer was washed sequentially with sodium bicarbonate solution and sodium chloride solution. Finally, the organic layer was dried with magnesium sulfate and concentrated to give 4-trifluoromethyl-1H-indole (4.5 g, 100% yield).
![EthenaMine, N,N-diMethyl-2-[2-nitro-6-(trifluoroMethyl)phenyl]-, (1E)-](/CAS/20150408/GIF/905274-06-8.gif)
905274-06-8
3 suppliers
inquiry

128562-95-8
111 suppliers
$27.00/100mg
Yield: 100%
Reaction Conditions:
with iron;acetic acidHeating / reflux;
Steps:
1.2
[0116] Step 2. 4-Trifluoromethyl-lH-indole[0117] To a solution of dimethyl-[2-(2-nitro-6-trifluoromethyl-phenyl)-vinyl]-amine (6.3 g, 24 mmol) in acetic acid (150 mL) was added iron powder (4.3 g, 77 mmol). The reaction was heated to reflux overnight under nitrogen. Upon cooling, the reaction mixture was diluted with 2.0 M HCl and the aqueous phase was extracted with ethyl acetate. The organic layer was neutralized with a saturated solution of potassium carbonate and extracted. Washed
References:
WYETH WO2008/60998, 2008, A1 Location in patent:Page/Page column 35-36
6656-49-1
175 suppliers
$6.00/1g

128562-95-8
111 suppliers
$27.00/100mg