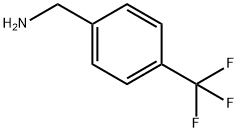
4-(Trifluoromethyl)benzylamine synthesis
- Product Name:4-(Trifluoromethyl)benzylamine
- CAS Number:3300-51-4
- Molecular formula:C8H8F3N
- Molecular Weight:175.15
![Benzaldehyde, 4-(trifluoromethyl)-, oxime, [C(Z)]-](/CAS/20211123/GIF/16939-49-4.gif)
16939-49-4

3300-51-4
General procedure for the synthesis of 4-(trifluoromethyl)benzylamine from the compound (CAS:16939-49-4): 5.00 g (26.4 mmol) of 4-trifluoromethylbenzaldehyde oxime was dissolved in methanol, followed by the addition of 4.0 g (109.7 mmol) of hydrogen chloride gas and 252 mg of the same catalyst as in Example 1, and the reaction was carried out with continuous stirring for 3 hours. The reaction was carried out at 10 atm hydrogen pressure and room temperature (about 25°C). Upon completion of the reaction, the catalyst was removed and ether was added to the reaction mixture and neutralized with aqueous sodium hydroxide. Analysis of the separated ether layer by gas chromatography confirmed the formation of 4-trifluoromethylbenzylamine in 93.6% yield.
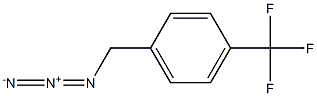
222716-19-0
2 suppliers
inquiry

3300-51-4
233 suppliers
$10.00/5G
Yield:3300-51-4 93%
Reaction Conditions:
with 1-phenylphospholane-1-oxide;1,3-diphenyl-disiloxane in tetrahydrofuran at 23; for 24 h;Staudinger Azide Reduction;Reagent/catalyst;Solvent;
Steps:
Additive-Free Catalytic Staudinger Reduction at Ambient Temperature; General Procedure
General procedure: To a 2-dram vial equipped with a stir bar were added all solid reagents(azide: 0.25 mmol, 1.0 equiv), 1a (4.5 mg, 0.025 mmol, 0.1equiv), THF (1 mL), all liquid reagents (azide: 0.25 mmol, 1.0 equiv),and then DPDS (57.5 mg, 0.25 mmol, 1.0 equiv). The reaction flaskwas sealed with a PTFE septum in the screwcap and the mixture wasstirred at 23 °C for 24 h. The mixture was diluted with acetone andthe vessel was washed with acetone (7.5 mL); this mixture was loadeddirectly onto silica gel (200 mg). Purification by flash column chromatography(EtOAc/hexanes, 40:60 with 1.5-5% Et3N) afforded amine products 4.
References:
Buonomo, Joseph A.;Cole, Malcolm S.;Eiden, Carter G.;Aldrich, Courtney C. [Synthesis,2020,vol. 52,# 23,p. 3583 - 3594]
455-19-6
413 suppliers
$6.00/5g

3300-51-4
233 suppliers
$10.00/5G
1891-90-3
143 suppliers
$9.00/5g

3300-51-4
233 suppliers
$10.00/5G
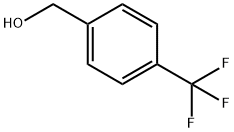
349-95-1
259 suppliers
$14.00/5g

3300-51-4
233 suppliers
$10.00/5G
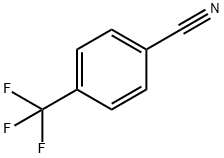
455-18-5
359 suppliers
$5.00/5g

3300-51-4
233 suppliers
$10.00/5G