4-(TRIFLUOROMETHYL)NICOTINAMIDE synthesis
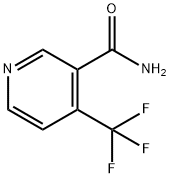
- Product Name:4-(TRIFLUOROMETHYL)NICOTINAMIDE
- CAS Number:158062-71-6
- Molecular formula:C7H5F3N2O
- Molecular Weight:190.12
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2Z)-](/CAS/20210305/GIF/533932-97-7.gif)
533932-97-7
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2E)-](/CAS/20210305/GIF/533932-96-6.gif)
533932-96-6

158062-71-6
Sodium hydroxide (600 mg, 15 mmol) was added as 3-[(4,4,4-trifluoro-3-oxo-1-butenyl)amino]-2-propenenitrile (mixture of IIa and IIb, 1.90 g, 10 mmol) and methanol (15 mL). The reaction mixture was heated to reflux for 6 hours. After completion of the reaction, the mixture was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography with hexane/acetone (1:1, v/v) as eluent to afford 4-(trifluoromethyl)nicotinamide (1.25 g, 65.6% yield). The product was characterized by 1H-NMR (200 MHz, DMSO-d6): δ 8.89 (1H, d, J = 5.1 Hz), 8.82 (1H, s), 8.18 (1H, brs), 7.85 (1H, brs), 7.81 (1H, d, J = 5.1 Hz).
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2Z)-](/CAS/20210305/GIF/533932-97-7.gif)
533932-97-7
0 suppliers
inquiry
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2E)-](/CAS/20210305/GIF/533932-96-6.gif)
533932-96-6
0 suppliers
inquiry

158062-71-6
160 suppliers
$13.43/250mgs:
Yield:158062-71-6 65.6%
Reaction Conditions:
with sodium hydroxide in methanol for 6 h;Heating / reflux;
Steps:
8 Reference Example 8; 4-Trifluoromethylnicotinamide (Compound VIIc, Step F)
(Reference Example 8) 4-Trifluoromethylnicotinamide (Compound VIIc, Step F) 3-[(4,4,4-Trifluoro-3-oxo-1-butenyl)amino]-2-propenenitrile (a mixture of IIa and IIb; 1.90g, 10 mmol) was dissolved in methanol (15 ml), and sodium hydroxide (600 mg, 15 mmol) was added.. The mixture was heated under reflux for 6 hours.. The reaction mixture was concentrated under reduced pressure.. The resulting residue was purified by silica gel column chromatography (a eluding solvent: hexane/acetone =1/1) to obtain 1.25 g (yield 65.6%) of the title compound.1H-NMR spectrum (200 MHz, DMSO-d6) δ (ppm) 8.89 (1H, d, J=5.1Hz), 8.82 (1H, s), 8.18 (1H, brs), 7.85 (1H, brs), 7.81 (1H, d, J=5.1Hz).
References:
Sankyo Agro Company, Limited EP1460071, 2004, A1 Location in patent:Page 39
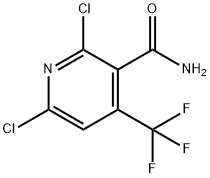
158063-67-3
88 suppliers
$35.00/250mg

158062-71-6
160 suppliers
$13.43/250mgs:
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2E)-](/CAS/20210305/GIF/533932-96-6.gif)
533932-96-6
0 suppliers
inquiry

158062-71-6
160 suppliers
$13.43/250mgs:
![2-Propenenitrile, 3-[(4,4,4-trifluoro-3-oxo-1-buten-1-yl)amino]-, (2Z)-](/CAS/20210305/GIF/533932-97-7.gif)
533932-97-7
0 suppliers
inquiry

158062-71-6
160 suppliers
$13.43/250mgs:
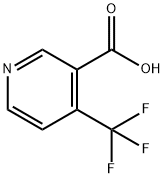
158063-66-2
389 suppliers
$5.00/250mg

158062-71-6
160 suppliers
$13.43/250mgs: