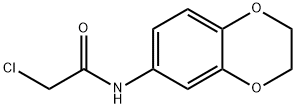
2-CHLORO-N-(2,3-DIHYDRO-BENZO[1,4]DIOXIN-6-YL)-ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(2,3-DIHYDRO-BENZO[1,4]DIOXIN-6-YL)-ACETAMIDE
- CAS Number:42477-07-6
- Molecular formula:C10H10ClNO3
- Molecular Weight:227.64

79-04-9
382 suppliers
$12.00/5g
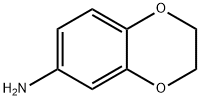
22013-33-8
195 suppliers
$8.00/250mg
![2-CHLORO-N-(2,3-DIHYDRO-BENZO[1,4]DIOXIN-6-YL)-ACETAMIDE](/CAS/GIF/42477-07-6.gif)
42477-07-6
44 suppliers
$57.50/250mg
Yield:42477-07-6 97%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 3 h;Inert atmosphere;
Steps:
General procedure D: Synthesis of acetamides 3n-w
General procedure: To a solution of a substituted aniline 24n-w (1 equiv.) and triethylamine (1.2 equiv.) in CH2Cl2 at 0 C was added chloroacetyl chloride (1.2 equiv.) over 15 minutes and the reaction stirred for 1 h at 0 C, followed by 2 h at r.t.. The salt was filtered off and washed with CH2Cl2 and EtOAc. This was dissolved in 1:1 MeOH/H2O, basified with sat. NaHCO3 and extracted with CH2Cl2 followed by drying with Na2SO4. The solvent was removed in vacuo to give the acetamides 3n-w.
References:
Haverkate, Natalie A.;van Rensburg, Michelle;Kumara, Sisira;Reynisson, Jóhannes;Leung, Euphemia;Pilkington, Lisa I.;Barker, David [Bioorganic and Medicinal Chemistry,2021,vol. 37] Location in patent:supporting information
16498-20-7
137 suppliers
$19.00/1g
![2-CHLORO-N-(2,3-DIHYDRO-BENZO[1,4]DIOXIN-6-YL)-ACETAMIDE](/CAS/GIF/42477-07-6.gif)
42477-07-6
44 suppliers
$57.50/250mg