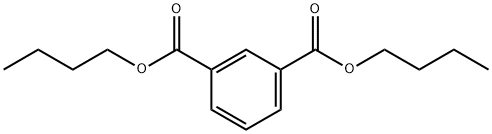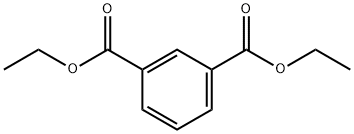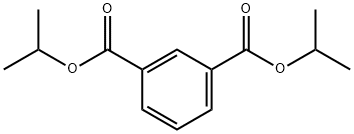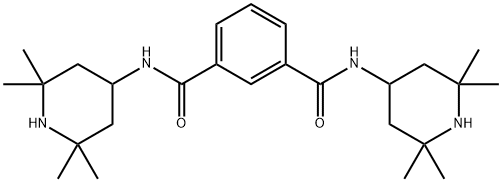
N,N'-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide synthesis
- Product Name:N,N'-Bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,3-benzenedicarboxamide
- CAS Number:42774-15-2
- Molecular formula:C26H42N4O2
- Molecular Weight:442.64
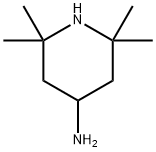
36768-62-4
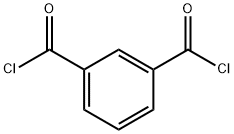
99-63-8

42774-15-2
An electric stirrer, reflux condenser and thermometer were assembled in each of the four reaction bottles. To each reaction vial was added 20.3 g of m-toluene dicarbonyl chloride, 36 g of 2,2,6,6-tetramethylpiperidinamine, 60 g of solvent, and 1.5 g of catalyst. the stirrer was started, heated to 110 °C and started to time, and the reaction time ranged from 4.5 to 10 hours. Upon completion of the reaction, the reaction mixture was slowly added to an alkaline aqueous solution under strong stirring to remove unreacted isophthaloyl chloride. Subsequently, the mixture was cooled to 15-20°C to promote crystallization. The crystallized product was collected by filtration to afford N1,N3-bis(2,2,6,6-tetramethylpiperidin-4-yl)isophthalamide. The light stabilizer had a yield of 97.38% and a melting point of 270-275 °C.

36768-62-4
288 suppliers
$7.00/10g
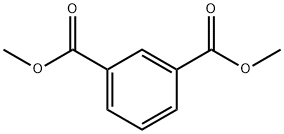
1459-93-4
347 suppliers
$12.60/100g

42774-15-2
114 suppliers
inquiry
Yield:42774-15-2 98.5%
Reaction Conditions:
with sodium methylate in methanol at 60 - 110; for 1.5 h;Inert atmosphere;Large scale;Temperature;
Steps:
1 Process Variant a
In a horizontal forced mixer that works at a Froudenumber of 2.16 and has been equipped with plowshares, adistillation colunm and a protective gas connection, 2.5 molof dimethyl isophthalate (m.p. 64-66° C.) and 5 mol of4-amino-2,2,6,6-tetramethylpiperidine (in liquid form atroom temperature) are homogenized with one another at atemperature of 60° C. under nitrogen until a monophasicliquid mixture forms. After the addition of 59.4 g of asodium methoxide solution (25% by weight in methanol),the reaction mixture is mixed at 110° C. for a duration of 90minutes. The alcohol from the catalyst formulation and thealcohol formed during the reaction are removed by distillation from the forced mixer. After the solid had been discharged and dried to constant weight, 1088.4 g (mass yield:9 8.5%) of a white powder were isolated.
References:
US2018/194727,2018,A1 Location in patent:Paragraph 0067; 0075; 0078