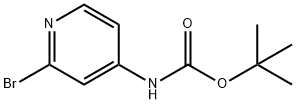
(2-BROMO-PYRIDIN-4-YL)CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:(2-BROMO-PYRIDIN-4-YL)CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:433711-95-6
- Molecular formula:C10H13BrN2O2
- Molecular Weight:273.13
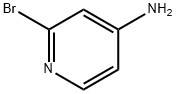
7598-35-8

24424-99-5

433711-95-6
General procedure for the synthesis of 4-tert-butoxycarbonylamino-2-bromopyridine from 4-amino-2-bromopyridine and di-tert-butyl dicarbonate: 2-bromopyridin-4-amine (100.0 g), triethylamine (Et3N, 64.8 g), and dichloromethane (DCM, 1.0 L) were added to the reaction vial and the reaction mixture was stirred for 15-30 min at 20-30 °C. Subsequently, 4-dimethylaminopyridine (DMAP, 3.5 g) was added to the reaction vial and di-tert-butyl dicarbonate ((Boc)2O, 416.3 g) was added slowly and dropwise. The reaction mixture was stirred continuously at 20-30 °C for 16 h, during which the progress of the reaction was monitored by HPLC. After completion of the reaction, the reaction mixture was washed with deionized water (2 L). The organic phase was separated and concentrated under reduced pressure to give the crude product as a yellow solid. The solid was stirred with methanol (MeOH, 300.0 mL) at 20-30 °C for 30 min and then filtered to give 4-tert-butoxycarbonylamino-2-bromopyridine (171.2 g) as an off-white solid.
6945-67-1
178 suppliers
$14.00/1g

24424-99-5
871 suppliers
$13.50/25G

433711-95-6
93 suppliers
$22.00/250mg
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 40; for 4 h;
Steps:
75.1 Step 1 Preparation of (2-bromopyridin-4-yl)carbamic acid tert-butyl ester
Take 2-bromo-4-aminopyridine (8.651g, 50.0mmol), triethylamine (15.179g, 150mmol)And dichloromethane (50mL) placed in the reaction flask, weigh di-tert-butyl carbonate(16.369g, 75.0mmol), added to the above reaction flask at 0 , add,The reaction was stirred at 40°C for 4h. After the reaction is complete, cool to room temperature,Add saturated sodium bicarbonate solution and dichloromethane to the reaction system, extract,Take the organic phase and dry with anhydrous sodium sulfate. After concentration under reduced pressure and purification by column chromatography, the title compound was obtained.
References:
Nanjing Shenghe Pharmaceutical Co., Ltd.;Wang Yong;Zhao Liwen;Wang Yazhou;Quan Xu;Liu Haixuan;Wang Xiaowei;Zhang Yan;Li Xue;Cao Chen;Guo Zhuang;Lv Kunzhi;Wang Hai;Zheng Guochuang CN111196804, 2020, A Location in patent:Paragraph 0783; 0785-0787

7598-35-8
365 suppliers
$6.00/250mg

24424-99-5
871 suppliers
$13.50/25G

433711-95-6
93 suppliers
$22.00/250mg