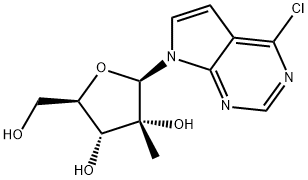
4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine synthesis
- Product Name:4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine
- CAS Number:443642-33-9
- Molecular formula:C12H14ClN3O4
- Molecular Weight:299.7103
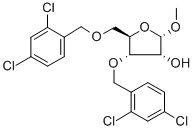
![7-[3,5-Bis-O-[(2,4-dichlorophenyl)methyl]-2-C-methyl-beta-D-ribofuranosyl]-4-chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/20211123/GIF/443642-32-8.gif)
443642-32-8
4 suppliers
inquiry
![4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180808/GIF/443642-33-9.gif)
443642-33-9
23 suppliers
inquiry
Yield:443642-33-9 84%
Reaction Conditions:
Stage #1: 4-chloro-7-[3,5-bis-O-(2,4-dichlorophenylmethyl)-2-C-methyl-β-D-ribofuranosyl]-7H-pyrrolo[2,3-d]pyrimidinewith boron trichloride in dichloromethane at -78 - -20;
Stage #2: with ammonia;water in methanol;dichloromethane at 0;
Steps:
23.2 Step 2.
Step 2. 4-Chloro-7-(2'-methyl-β-D-ribofuranosyl)-7H -pyrrolo[2,3-d]pyrimidine To the solution of the product from Step 1 (10.0 g, 16.19 mmol) in dichloromethane (440 mL) at -78° C. was added boron trichloride (1M in dichloromethane) (157 mL, 157.0 mmol) dropwise over 30 minutes. The mixture was stirred at -78° C. for 2 hours then at -20° C. overnight. The reaction was quenched with dichloromethane/methanol 1:1 (420 mL) and neutralized at 0° C. with aqueous ammonia. The solid was filtered, washed with dichloromethane/methanol 1:1 and the combined extracts evaporated in vacuo. The residue was purified on silica gel column with dichloromethane/methanol (10:1 v/v) as eluent. Fractions containing product were combined and concentrated to yield 4.1 g (84%) of the deprotected nucleoside; MS: 300.08 (M+1); 1H NMR (D2O): δ 8.32 (s, 1H), 7.57 (d, 1H, J=3.6 Hz), 6.56 (d, 1H, J=3.6 Hz), 6.17 (s, 1H), 4.0-3.5 (m, 4H), 0.65 (s, 3H). cl Step 3. 4-Amino-7-(2'-methyl-β-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidine The product from Step 2 (1.1 g, 3.68 mmol) was placed in an autoclave pressure bomb and liquid ammonia was added (10 mL) at -78° C. The vessel was sealed and heated to 85° C. for 24 hours. The vessel was cooled back to -78° C., opened and the ammonia was allowed to evaporate. The residue was taken up in a small amount of methanol and plated onto a glass filter column containing a small pad of silica gel. The methanol was allowed to evaporate under vacuum and the product was eluded by ramping to 20% methanol in dichloromethane to give 1.0 g (97%) of a light yellow powder; 1H NMR (CD3OD): δ 8.06 (s, 1H), 7.48 (d, 1H, J=3.6 Hz), 6.60 (d, 1H, J=3.6 Hz), 6.21 (s, 1H), 4.13-3.85 (m, 4H), 0.81 (s, 3H); MS: 281.14 (M+1).
References:
US2009/48189,2009,A1 Location in patent:Page/Page column 45
168427-35-8
57 suppliers
$45.00/250mg
![4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180808/GIF/443642-33-9.gif)
443642-33-9
23 suppliers
inquiry
![1-O-Methyl-3,5-bis-O-[(2,4-dichlorophenyl)methyl]-alpha-D-erthro-pentofuranoside-2-ulose](/CAS/GIF/443642-30-6.gif)
443642-30-6
52 suppliers
$75.00/50mg
![4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180808/GIF/443642-33-9.gif)
443642-33-9
23 suppliers
inquiry
![1-O-Methyl-3,5-bis-O-[(2,4-dichlorophenyl)methyl]-2-C-methyl-alpha-D-ribofuranoside](/CAS/GIF/443642-31-7.gif)
443642-31-7
57 suppliers
$60.00/100mg
![4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180808/GIF/443642-33-9.gif)
443642-33-9
23 suppliers
inquiry
741686-50-0
0 suppliers
inquiry
![4-Chloro-7-(2-C-methyl-beta-D-ribofuranosyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/20180808/GIF/443642-33-9.gif)
443642-33-9
23 suppliers
inquiry