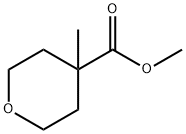
2H-Pyran-4-carboxylicacid,tetrahydro-4-methyl-,methylester(9CI) synthesis
- Product Name:2H-Pyran-4-carboxylicacid,tetrahydro-4-methyl-,methylester(9CI)
- CAS Number:443912-70-7
- Molecular formula:C8H14O3
- Molecular Weight:158.2
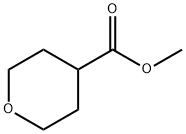
110238-91-0

74-88-4

443912-70-7
At -78 °C, 22.1 mL of n-butyllithium (35.4 mmol, 1.6 M hexane solution) was slowly added dropwise to a solution of 4.91 mL (35.0 mmol) of diisopropylamine in 45 mL of tetrahydrofuran, and the mixture was stirred at -78 °C for 10 minutes, followed by 25 minutes at 0 °C. Then, 5.00 g (34.7 mmol) of methyl tetrahydro-2H-pyran-4-carboxylate in a solution in 45 mL of tetrahydrofuran was added at -78 °C and the mixture continued to be stirred at -78 °C for 15 minutes. Next, 2.16 mL (34.7 mmol) of iodomethane was added dropwise at -78 °C and the reaction mixture was slowly warmed to -25 °C and stirred overnight at room temperature. Upon completion of the reaction, the reaction was terminated by the addition of 80 mL of 0.1 N hydrochloric acid and the organic and aqueous phases were separated. The aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, dried with magnesium sulfate, filtered and concentrated under reduced pressure. The resulting suspension was ground with 20 mL of methyl tert-butyl ether, the precipitate was extracted, and the mother liquor was concentrated under reduced pressure to obtain methyl 4-methyl-tetrahydro-2H-pyran-4-carboxylate. Yield: 5.88 g (purity 95%, quantitative yield).1H NMR (400 MHz, DMSO-d6) δ (ppm): 3.70-3.64 (m, 2H), 3.64 (s, 3H), 3.37-3.30 (m, 2H), 1.94-1.88 (m, 2H), 1.45-1.37 (m, 2H), 1.16 (s 3H).

110238-91-0
213 suppliers
$13.00/5g

74-88-4
357 suppliers
$15.00/10g

443912-70-7
65 suppliers
$50.00/250mg
Yield:443912-70-7 100%
Reaction Conditions:
Stage #1: tetrahydropyran-4-carboxylic acid methyl esterwith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;hexane at -78 - 0; for 0.75 h;
Stage #2: iodomethane in tetrahydrofuran;hexane at -78 - 20;
Steps:
64.1A Methy I 4-methy ltetrahydro-2H-pyran -4-carboxy late
[1749] At -78° C., 22.1 ml ofn-butyllithium (35.4 mmol,1.6M in hexane) were slowly added dropwise to a solution of4.91 ml (35.0 mmol) of diisopropylamine in 45 ml oftetrahydrofuran,and the mixture was stirred at -78° C. for another 10min and at oo C. for another 25 min. Subsequently, at -78° C.,a solution of 5.00 g (34.7 mmol) of methyl tetrahydro-2Hpyran-4-carboxylate in 45 ml oftetrahydrofuran was added,and the mixture was stirred at -78° C. for another 15 min andat oo C. for another 30 min. At -78° C., 2.16 ml (34.7 mmol)of methyl iodide were added dropwise, and the reaction mixturewas slowly warmed to -25° C. and then to room temperatureovernight. The reaction was terminated by additionof 80 ml of O.lN hydrochloric acid, and the phases wereseparated. The aqueous phase was extracted twice with ethylacetate, the combined organic phases were dried over magnesiumsulphate and filtered and the solvent was removedunder reduced pressure. The resulting suspension was trituratedwith 20 ml of methyl tert-butyl ether, the precipitate wasfiltered off with suction and the mother liquor was concentratedunder reduced pressure, giving the title compound.Yield: 5.88 g (purity 95%, quant.)[1750] 1H-NMR (400 MHz, DMSO-d6): o [ppm]=3.70-3.64 (m, 2H), 3.64 (s, 3H), 3.37-3.30 (m, 2H), 1.94-1.88 (m,2H), 1.45-1.37 (m, 2H), 1.16 (s, 3H).
References:
US2016/52884,2016,A1 Location in patent:Paragraph 1748-1750

74-88-4
357 suppliers
$15.00/10g

443912-70-7
65 suppliers
$50.00/250mg