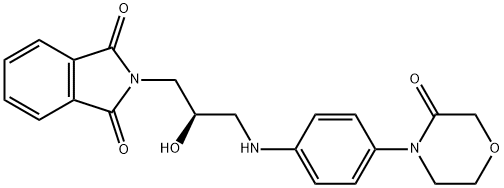
2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione synthesis
- Product Name:2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione
- CAS Number:446292-07-5
- Molecular formula:C21H21N3O5
- Molecular Weight:395.41
![4-[4-(N-(3-chloro-(2R)-2-hydroxy-1-propyl)aMino)phenyl]Morpholin-3-one](/CAS/20150408/GIF/1252018-10-2.gif)
1252018-10-2
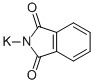
1074-82-4
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
Example 3: Preparation of (R)-2-(2-hydroxy-3-((4-(3-oxomorpholinyl)phenyl)amino)propyl)isoindoline-1,3-dione (7a): phthalimide potassium salt (7.16 g, 38.632 mmol) was added in a single addition to a mechanically stirred N,N-dimethylformamide (DMF, 60 mL) solution of 4-[4-(N-(3-chloro-2R-hydroxy-1-propyl)amino)phenyl]morpholine -3-one (10 g, 35.119 mmol) in a solution of N,N-dimethylformamide (DMF, 60 mL). After stirring the suspension at room temperature, it was heated to 100 °C and stirred continuously at this temperature for 3 hours. After the reaction was completed, the mixture was cooled to room temperature. Water (60 mL) was added and the suspension continued to be stirred for 15 minutes. Subsequently, the suspension was filtered through a Brinell funnel and the solid product was collected. The solid was washed with distilled water (2 x 40 mL) and dried under vacuum at 50 °C for 10 h to afford the target product (R)-2-(2-hydroxy-3-((4-(3-oxomorpholinyl)phenyl)amino)propyl)isoindoline-1,3-dione (12.55 g, 93% yield) as a white crystalline solid.1H NMR (400 MHz, DMSO-d6) δ. 2.99-3.05 (m, 1H), 3.14-3.2 (m, 1H), 3.59-3.69 (m, 4H), 3.91-3.94 (m, 2H), 3.97-4.05 (m, 1H), 4.14 (s, 2H), 5.16 (d, J = 5.2 Hz, 1H), 5.66 (t, J = 6.0 Hz, 1H), 6.61 (d, J = 6.0 Hz, 1H), 6.61 (d, J = 6.0 Hz, 1H). 6.61 (d, J = 8.7 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 7.82-7.88 (m, 4H).
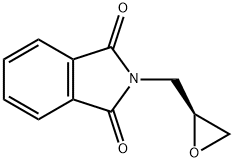
161596-47-0
592 suppliers
$6.00/5g
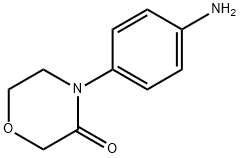
438056-69-0
613 suppliers
$10.00/5g
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
261 suppliers
inquiry
Yield:446292-07-5 95%
Reaction Conditions:
in ethanol;water at 20; for 15 h;Solvent;Temperature;
Steps:
4-9 Example 4: Preparation of Compound C
Add 96g Compound A (made in accordance with the example of Patent Document CN100430384C, HPLC purity 96.0%), 960mL 95% (v/v) ethanol aqueous solution and 120g Compound B (made according to the method of Example 1) to the reaction flask, and reflux to react 15 hours, cooled to 20 °C, filtered, washed with 200 mL of 95% ethanol aqueous solution, and dried under vacuum at 70 ° C for 5 hours to obtain 188 g of compound C, off-white solid, molar yield: 95%, HPLC purity: 95.0%, impurity E content: 2.8 %, Ee value: 96.2%.
References:
Zhejiang Haixiang Pharmaceutical Co., Ltd.;Shanghai Haixiang Pharmaceutical Co., Ltd.;Tang Fanghui;Liang Chao;Ni Zhizhong;Zhao Fulu;Li Hongming CN111039937, 2020, A Location in patent:Paragraph 0066-0082
![4-[4-(N-(3-chloro-(2R)-2-hydroxy-1-propyl)aMino)phenyl]Morpholin-3-one](/CAS/20150408/GIF/1252018-10-2.gif)
1252018-10-2
51 suppliers
inquiry

1074-82-4
502 suppliers
$5.00/25g
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
261 suppliers
inquiry
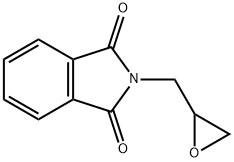
5455-98-1
176 suppliers
$25.00/5g

438056-69-0
613 suppliers
$10.00/5g
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
261 suppliers
inquiry
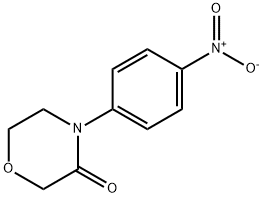
446292-04-2
174 suppliers
inquiry
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
261 suppliers
inquiry
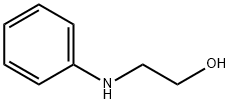
122-98-5
315 suppliers
$5.00/5 g
![2-[(2R)-2-Hydroxy-3-[[4-(3-oxo-4-Morpho linyl)phenyl]aMino]propyl]-1H-isoindole-1 ,3(2H)-dione](/CAS/GIF/446292-07-5.gif)
446292-07-5
261 suppliers
inquiry