
[2-oxo-2-(3-pyridinyl)ethyl]-carbamic acid 1,1-dimethylethyl ester synthesis
- Product Name:[2-oxo-2-(3-pyridinyl)ethyl]-carbamic acid 1,1-dimethylethyl ester
- CAS Number:473693-42-4
- Molecular formula:C12H16N2O3
- Molecular Weight:236.27
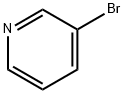
626-55-1
581 suppliers
$5.00/5/ G
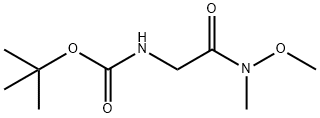
121505-93-9
142 suppliers
$6.00/1g
![[2-oxo-2-(3-pyridinyl)ethyl]-carbamic acid 1,1-dimethylethyl ester](/CAS/20180703/GIF/473693-42-4.gif)
473693-42-4
5 suppliers
inquiry
Yield:473693-42-4 66%
Reaction Conditions:
Stage #1: 3-Bromopyridinewith isopropylmagnesium chloride lithium chloride in tetrahydrofuran at 20; for 1 h;
Stage #2: N-(tert-butoxycarbonyl)glycine N'-methoxy-N'-methylamide in tetrahydrofuran at -15 - 20;
Steps:
14.a
To a solution of 3-bromopyridine (1.21 mL, 12.6 mmol) in 15 mL of dry THF was added isopropylmagnesium chloride (2 M in THF, 6.3 mL, 12.6 mmol) at room temperature under N2. After 45 min. , in a separate flask, isopropylmagnesium chloride (4.9 mL, 9.8 mmol) was added to a cooled (-15 to-10 °C) slurry of N-(tert-butoxycarbonyl) glycine N'- methoxy-X-methylamide (2.18 g, 10.0 mmol) in 15 mL of dry THF under N2. After the Br- Mg exchange reaction had stirred for a total of 1 hr, the resulting mixture was added to the Weinreb amide anion solution. After the entire contents had been added, the reaction mixture was allowed to warm to room temperature and stirred overnight. The mixture was then partitioned between EtOAc and water. The layers were separated and the aqueous layer was extracted with EtOAc. The organic extracts were combined, dried over MgS04, filtered and concentrated in vacuo. The residue was chromatographed on silica gel (100% Hexane to 25% EtOAc/Hexane gradient) to give 1.57 g of a white solid as desired product (66%). 1H- NMR: 300MHz, CDC13 8 9.17 (m, 1H) ; 8.82 (m, 1H) ; 8.23 (m, 1H); 7.44 (m, 1H) ; 5.45 (br s, 1H) ; 4.66 (d, 2H); 1.48 (s, 9H).
References:
WO2005/61510,2005,A1 Location in patent:Page/Page column 24
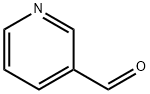
500-22-1
510 suppliers
$6.00/5g
![[2-oxo-2-(3-pyridinyl)ethyl]-carbamic acid 1,1-dimethylethyl ester](/CAS/20180703/GIF/473693-42-4.gif)
473693-42-4
5 suppliers
inquiry

101861-30-7
3 suppliers
inquiry
![[2-oxo-2-(3-pyridinyl)ethyl]-carbamic acid 1,1-dimethylethyl ester](/CAS/20180703/GIF/473693-42-4.gif)
473693-42-4
5 suppliers
inquiry