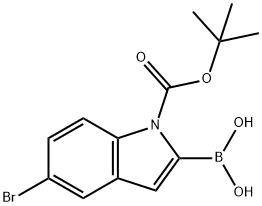
5-Bromo-1-(tert-butoxycarbonyl)-1H-indol-2-ylboronic acid synthesis
- Product Name:5-Bromo-1-(tert-butoxycarbonyl)-1H-indol-2-ylboronic acid
- CAS Number:475102-13-7
- Molecular formula:C13H15BBrNO4
- Molecular Weight:339.98
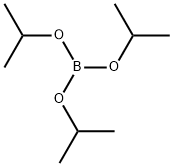
5419-55-6
387 suppliers
$12.00/5g
7732-18-5
507 suppliers
$12.69/100ml
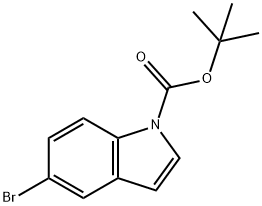
182344-70-3
100 suppliers
$9.00/250mg

475102-13-7
129 suppliers
$11.00/1g
Yield: 52%
Reaction Conditions:
Stage #1:Triisopropyl borate;5-bromo-indole-1-carboxylic acid tert-butyl ester with lithium diisopropyl amide in tetrahydrofuran at -5 - 0; for 1 h;
Stage #2:water in tetrahydrofuran
Steps:
(5-bromo-1-(tert-butoxycarbonyl)-1H-indol-2-yl)boronic acid (U3)
According to Scheme 3, step ii:
to the solution of tert-butyl 5-bromo-1H-indole-1-carboxylate (2 g, 6.75 mmol) in THF (10 mL) was added triisopropyl borate (2.54 g, 13.5 mmol, 3.11 mL).
The solution was cooled to -5°C, LDA (2 M, 5.06 mL) was added.
The mixture was stirred at 0°C for 1 hr.
For this example, the same experiment was performed three times.
The combined mixture was poured into H2O (100 mL).
Then extracted with EtOAc (100 mL x 3), the organic phase was combined and concentrated under vacuum.
The residue was washed with CH3CN (40 mL).
The mixture was filtered, the collected solid was dried under vacuum to give intermediate U3 (5 g, 96% purity, yield: 52%) as a white solid. 1HNMR (MeOD): δ ppm HNMR: 400 MHz, MeOD: δ 8.02 (d, J = 8.8 Hz, 1H), 7.70 (s, 1H), 7.37-7.40 (m, 1H), 6.60 (s, 1H), 1.67 (s, 9H).
References:
GenKyoTex Suisse SA;MACHIN, Peter;CHAMBERS, Mark;HODGES, Alastair;SHARPE, Andrew;WISHART, Grant;PERRY, Benjamin;CELANIRE, Sylvain;HEITZ, Freddy EP3628669, 2020, A1 Location in patent:Paragraph 0299; 0300

182344-70-3
100 suppliers
$9.00/250mg

475102-13-7
129 suppliers
$11.00/1g
10075-50-0
689 suppliers
$5.00/10g

475102-13-7
129 suppliers
$11.00/1g

24424-99-5
869 suppliers
$13.50/25G

475102-13-7
129 suppliers
$11.00/1g