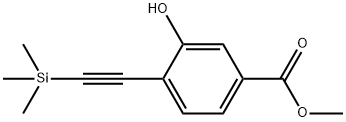
Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate synthesis
- Product Name:Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate
- CAS Number:478169-68-5
- Molecular formula:C13H16O3Si
- Molecular Weight:248.35
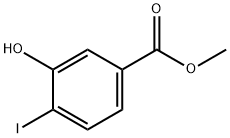
157942-12-6
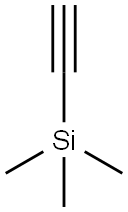
1066-54-2

478169-68-5
Methyl 3-hydroxy-4-iodobenzoate (5.22 g, 18.8 mmol) and trimethylsilylacetylene (3.71 mL, 26.3 mmol) were used as raw materials, which were added under nitrogen protection along with bis(triphenylphosphine)palladium dichloride (386 mg, 0.55 mmol) and cuprous iodide (54 mg, 0.28 mmol) in a dry THF (20 mL ) and CHCl3 (40 mL) in a solvent mixture. Subsequently, triethylamine (8.14 mL, 58.4 mmol) was added to the reaction system and the mixture was heated to 50 °C for 4 hours. After completion of the reaction, the reaction mixture was diluted with CHCl3 (60 mL), washed sequentially with 5% HCl (2 x 40 mL), and the organic phase was dried with MgSO4 and concentrated to give a brown oily solid (8.31 g). The crude product was purified by column chromatography (Biotage standard column, 90 g silica gel), eluting first with 10% EtOAc/hexane (1 L) and then with 15% EtOAc/hexane (1 L). The fraction containing the target product was collected and concentrated to give methyl 3-hydroxy-4-((trimethylsilyl)alkynyl)benzoate (4.22 g, 91% yield) as a yellow solid. High-resolution mass spectrometry (FAB) analysis results: C13H16O3Si [M+H]+ calculated value 249.0947, measured value 249.0947.

157942-12-6
119 suppliers
$9.00/250mg

1066-54-2
473 suppliers
$9.00/10g

478169-68-5
42 suppliers
$36.00/100mg
Yield:478169-68-5 96.2%
Reaction Conditions:
with copper (I) iodide;triethylamine;triphenylphosphine in dichloromethane at 20 - 27; for 0.8 h;Inert atmosphere;Temperature;
Steps:
1.1-1.4; 2.1-2.3; 3.1-3.3
(2) in 100ml reaction flask, add methylene dichloride 36g, 3 g of methyl 4-iodo-3-hydroxybenzoate, 3.5 g of triethylamine, Cuprous iodide 0.20g, triphenylphosphine 0.130g, after nitrogen replacement, Add 0.10 g of palladium-supported titanium dioxide hollow nano-microsphere catalyst; Control the temperature in the kettle at 20-27°C, add 1.33g of trimethylsilyl acetylene dropwise to the reactor, Incubate for 48min; HPLC monitors the reaction end point to confirm that the middle control is qualified; 30ml of water was added to the 100ml reaction flask, the temperature was lowered to 10-15°C, and the reaction solution was transferred. 6.6g of 5M nitric acid was added dropwise, stirred for 30min, and the pH of the aqueous phase was detected as 2.69. Let stand, carry out liquid separation, retain the organic phase, and the organic phase is concentrated and distilled under reduced pressure to obtain the crude product; Add 30 ml of methanol to the crude product in the distillation flask, and after stirring, Continue to distill under reduced pressure to obtain the product. The yield is about 96.2%, and the product purity is 98.874%. Its chromatogram is shown in Figure 6. (3) The aqueous phase obtained by liquid separation is filtered to obtain a palladium-supported titanium dioxide hollow nano-microsphere catalyst, placed in methanol, and a Soxhlet extractor is used at 55-60° C., Heating with stirring, reflux washing for 5h, activation, and regeneration.
References:
CN114315885,2022,A Location in patent:Paragraph 0028; 0043-0061