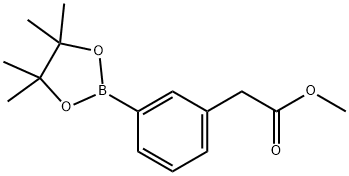
Methyl 2-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate synthesis
- Product Name:Methyl 2-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate
- CAS Number:478375-42-7
- Molecular formula:C15H21BO4
- Molecular Weight:276.14
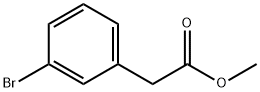
150529-73-0

73183-34-3

478375-42-7
Step 1: Synthesis of methyl 2-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)acetate Methyl 2-(3-bromophenyl)acetate (5.00 g, 21.8 mmol), bis(pinacolato)diboron (11.1 g, 43.7 mmol), and potassium acetate (6.43 g, 65.5 mmol) were dissolved in dioxane (43.7 mL) and degassed by bubbling nitrogen. PdCl2 (dppf)-CH2Cl2 adduct (0.446 g, 0.546 mmol) was added and the reaction mixture was heated to 85 °C and stirred for 4 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (30 mL) and filtered through diatomaceous earth. The organic layer was separated, washed with brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography (220 g silica gel column, elution gradient 0 to 40% ethyl acetate/hexanes) to afford the target product 3-(2-methoxy-2-oxoethyl)phenylboronic acid pinacol ester (6.00 g, 100%) as a colorless oil. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.74 (s, 1H), 7.72 (s, 1H), 7.40 (t, J=1.7 Hz, 1H), 7.38-7.33 (m, 1H), 3.70 (s, 3H), 3.65 (s, 2H), 1.36 (s, 12H). lcmS (M+H) = 277.3; HPLC retention time = 0.99 min (column: BEH C18 2.1×50 mm; mobile phase A: water containing 0.05% TFA; mobile phase B: acetonitrile containing 0.05% TFA; gradient: 2-8% B in 1.6 min; flow rate: 0.8 mL/min).

150529-73-0
158 suppliers
$6.00/1g

73183-34-3
583 suppliers
$6.00/5g

478375-42-7
59 suppliers
$67.00/250mg
Yield:478375-42-7 100%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 85; for 4 h;Inert atmosphere;
Steps:
255.1 Step 1: Methyl 2-(3-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)phenyl)acetate
Step 1: Methyl 2-(3-(4,4,5,5-tetramethyl- 1 ,3,2-dioxaborolan-2-yl)phenyl)acetateA suspension of methyl 2-(3-bromophenyl)acetate (5.00 g, 21.8 mmol), bis(pinacolato)diboron (11.1 g, 43.7 mmol) and potassium acetate (6.43 g, 65.5 mmol) in dioxane (43.7 ml) was degassed with bubbling nitrogen. PdC12(dppf)-CH2C12 adduct (0.446 g, 0.546 mmol) was added, and the reaction was heated to 85 °C for 4 h. The reaction was diluted with ethyl acetate (30 mL) and filtered through Celite. The organiclayer was washed with brine, separated, and dried with sodium sulfate. The solvent was evaporated, and the residue was purified by column chromatography on silica gel (220 g column, gradient from 0% to 40% EtOAc/hexanes) to give the title compound (6.00 g, 100%) as a colorless oil. ‘H NMR (400MHz, CDC13) ? 7.74 (s, 1H), 7.72 (s, 1H), 7.40 (t, J=1.7 Hz, 1H), 7.38-7.33 (m, 1H), 3.70 (s, 3H), 3.65 (s, 2H), 1.36 (s, 12H); LCMS(M+H) = 277.3; HPLC RT = 0.99 mm (Column: BEH C18 2.1 x 50 mm; Mobile Phase A: Water with 0.05% TFA; Mobile Phase B: Acetonitrile with 0.05% TFA; Gradient: 2- 98% B over 1.6 mm; Flow: 0.8 mL/min).
References:
WO2015/100282,2015,A1 Location in patent:Page/Page column 279

73183-34-3
583 suppliers
$6.00/5g
147283-87-2
2 suppliers
inquiry

478375-42-7
59 suppliers
$67.00/250mg
1878-67-7
338 suppliers
$5.00/5g

478375-42-7
59 suppliers
$67.00/250mg
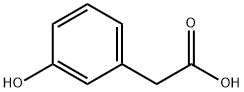
621-37-4
320 suppliers
$6.00/1g

478375-42-7
59 suppliers
$67.00/250mg
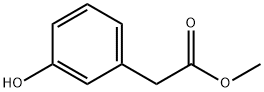
42058-59-3
122 suppliers
$15.00/1g

478375-42-7
59 suppliers
$67.00/250mg