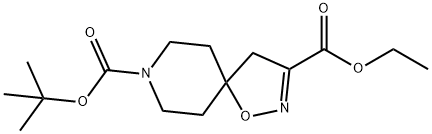
8-tert-butyl 3-ethyl 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-3,8-dicarboxylate synthesis
- Product Name:8-tert-butyl 3-ethyl 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-3,8-dicarboxylate
- CAS Number:479636-65-2
- Molecular formula:C15H24N2O5
- Molecular Weight:312.36
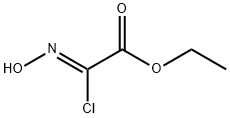
95080-93-6
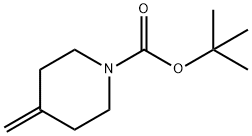
159635-49-1
![8-tert-butyl 3-ethyl 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-3,8-dicarboxylate](/CAS/20180703/GIF/479636-65-2.gif)
479636-65-2
Compound 7-a (1.0 g, 5.1 mmol) and sodium bicarbonate (2.1 g, 25 mmol) were dissolved in ethyl acetate (10 mL). After the mixture was cooled to 0 °C, ethyl (2Z)-2-chloro-2-(hydroxyimino)acetate (1.2 g, 7.6 mmol, prepared with reference to the literature method, Tetrahedron Letters, 2011, 52(43), 5656-5658) was slowly added. The reaction mixture was stirred at room temperature for 48 hours. After completion of the reaction, it was diluted by adding distilled water (100 mL) and extracted with ethyl acetate. The organic layers were combined, washed sequentially with distilled water and saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, 5:95→3:7) to afford the target product 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-3,8-dicarboxylic acid-8-tert-butyl ester-3-ethyl ester 7-b (1.4 g, 93% yield) as a yellow solid.

95080-93-6
1 suppliers
inquiry

159635-49-1
17 suppliers
$5.00/250mg
![8-tert-butyl 3-ethyl 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-3,8-dicarboxylate](/CAS/20180703/GIF/479636-65-2.gif)
479636-65-2
31 suppliers
$75.00/100mg
Yield:479636-65-2 93%
Reaction Conditions:
with sodium hydrogencarbonate in ethyl acetate at 0 - 20; for 48 h;
Steps:
1-7.2 (Step 2) Preparation of 8-tert-butyl-3-ethyl-1-oxa-2,8-diazaspiro [4.5] dec-2-ene-3,8-dicarboxylate
Compound 7-a (1.0 g, 5.1 mmol) and sodium hydrogencarbonate (2.1 g, 0.025 mol) were dissolved in ethyl acetate (10 mL) After cooling, the mixture was cooled to 0 ° C and ethyl (2Z) -2-chloro-2- (hydroxyimino) acetate (prepared according to a known method (Tetrahedron Letters, 2011, 52 (43), 5656-5658 1.2 g, 7.6 mmol) was added, And the mixture was stirred at room temperature for 48 hours. After completion of the reaction, distilled water (100 mL) was added and the mixture was extracted with ethyl acetate. The organic layer was washed with distilled water and saturated brine, dried over anhydrous sodium sulfate and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate: n-hexane = 5: 95 → 3: 7) to give the title compound 7-b (1.4 g, 93%) as a yellow solid.
References:
KR101798840,2017,B1 Location in patent:Paragraph 0483-0484; 0488-0489