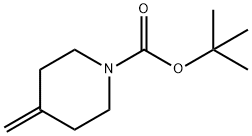
tert-Butyl 4-methylenepiperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-methylenepiperidine-1-carboxylate
- CAS Number:159635-49-1
- Molecular formula:C11H19NO2
- Molecular Weight:197.27
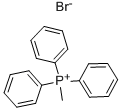
1779-49-3

79099-07-3

159635-49-1
Generalized method: Step 1: Synthesis of tert-butyl 4-methylene piperidine-1-carboxylate To a suspension of methyltriphenylphosphonium bromide (36.3 g, 101.6 mmol, 1.35 eq.) in dry ether (300 mL) was added potassium tert-butoxide (1 g, 98 mmol, 1.3 eq.) in a single addition at 0°C under nitrogen protection. The mixture was heated to reflux and stirred for 2 hours. Subsequently, the reaction mixture was cooled to 0 °C using an external ice bath and a solution of N-tert-butoxycarbonyl-4-piperidone (15 g, 75.3 mmol, 1.0 equiv) in ether (60 mL) was added dropwise. The mixture was slowly warmed to room temperature and stirring was continued at this temperature for 1 hour. After that, the mixture was heated to reflux again and stirred overnight (16 hours). Upon completion of the reaction, the mixture was cooled to room temperature, hexane (300 mL) was added, stirred for 10 minutes, filtered, and eluted with a solvent mixture of hexane/EtOAc (100/100 mL). The organic phases were combined and concentrated to give the crude product, which was finally purified by a CombiFlash system (100 g silica gel column, EtOAc/Hexane gradient elution, 0-30%) to give 14 g (94% yield) of tert-butyl 4-methylene piperidine-1-carboxylate as a colorless oil. 1H NMR (400 MHz, CDCl3): δ 4.74 (s, 2H), 3.42 (t, 4H), 2.18 (t, 4H), 1.47 (s, 9H).

79099-07-3
0 suppliers
$5.00/5g

159635-49-1
17 suppliers
$5.00/250mg
Yield:159635-49-1 99%
Reaction Conditions:
Stage #1: Methyltriphenylphosphonium bromidewith n-butyllithium in tetrahydrofuran at -78; for 1 h;
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one in tetrahydrofuran at -78 - 20;
Steps:
65
[0242] To a solution of methyl triphenylphosphonium bromide (5.4g, 15.05mmol) in THF (lOOmL) was added slowly butyl lithium (2M, 15.05mmol) at -78°C. The mixture was allowed to stir for one hour and N-Boc-piperidinone (2g, 10.03mmol) was added. The mixture was warmed to room temperature and stirred overnight. The THF was evaporatedand the residue was partitioned between between water and ethyl acetate. The organic layer was washed with brine and dried over sodium sulphate, filtered and concentrated. The compound was purified by column chromatography (30% Hexanes/EtOAc) to provide the title compound (1.96g, 99%).
References:
WO2006/20879,2006,A1 Location in patent:Page/Page column 101-102
2065-66-9
325 suppliers
$5.00/5g

79099-07-3
0 suppliers
$5.00/5g

159635-49-1
17 suppliers
$5.00/250mg

1779-49-3
623 suppliers
$5.00/10g

79099-07-3
0 suppliers
$5.00/5g

159635-49-1
17 suppliers
$5.00/250mg
![1-methyl-2-(methylsulfonyl)-1H-benzo[d]imidazole](/CAS/20180703/GIF/61078-14-6.gif)
61078-14-6
45 suppliers
$60.00/50mg

79099-07-3
0 suppliers
$5.00/5g

159635-49-1
17 suppliers
$5.00/250mg
873869-19-3
4 suppliers
inquiry

79099-07-3
0 suppliers
$5.00/5g

159635-49-1
17 suppliers
$5.00/250mg