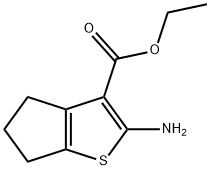
2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:4815-29-6
- Molecular formula:C10H13NO2S
- Molecular Weight:211.28

120-92-3

105-56-6
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
GENERAL METHOD: Cyclopentanone (1.06 mL, 10 mmol), ethyl cyanoacetate (1.15 mL, 10 mmol), morpholine (0.90 mL, 10 mmol), and sulfur powder (0.32 g, 10 mmol) were dissolved in ethanol (10 mL) with stirring and refluxed overnight. Upon completion of the reaction, the reaction mixture was cooled to room temperature and the solvent was subsequently removed under vacuum. The crude product was washed with cold ethanol, filtered through a sintered funnel and dried under vacuum. The dried crude product was dissolved in dichloromethane and washed with brine. The organic layer was separated and concentrated under low vacuum to afford the target compound ethyl 2-amino-5,6-dihydro-4H-cyclopenta[b]thiophene-3-carboxylate (2a), yield: 73% (1.83 g).

105-56-6
462 suppliers
$10.00/5ml
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
129 suppliers
$6.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 28 percent / CH3COONH4 / acetic acid; benzene / 3.5 h / Heating
2: 37 percent / S, morpholine / ethanol / 15 h / Ambient temperature
References:
Peet, Norton P.;Sunder, Shyam;Barbuch, Robert J.;Vinogradoff, Anna P. [Journal of Heterocyclic Chemistry,1986,vol. 23,p. 129 - 134]

120-92-3
427 suppliers
$11.00/25g

105-56-6
462 suppliers
$10.00/5ml
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
129 suppliers
$6.00/250mg
5407-83-0
20 suppliers
$45.00/50mg
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
129 suppliers
$6.00/250mg

120-92-3
427 suppliers
$11.00/25g
53841-07-9
29 suppliers
inquiry
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
129 suppliers
$6.00/250mg

120-92-3
427 suppliers
$11.00/25g
![2-AMINO-5,6-DIHYDRO-4H-CYCLOPENTA[B]THIOPHENE-3-CARBOXYLIC ACID ETHYL ESTER](/CAS/GIF/4815-29-6.gif)
4815-29-6
129 suppliers
$6.00/250mg