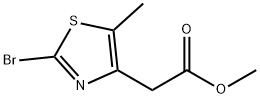
4-Thiazoleaceticacid,2-bromo-5-methyl-,methylester(9CI) synthesis
- Product Name:4-Thiazoleaceticacid,2-bromo-5-methyl-,methylester(9CI)
- CAS Number:496062-15-8
- Molecular formula:C7H8BrNO2S
- Molecular Weight:250.11
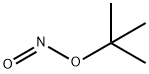
540-80-7
358 suppliers
$29.00/25g
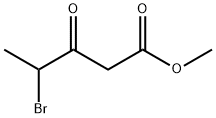
105983-77-5
43 suppliers
$130.50/1g

496062-15-8
12 suppliers
inquiry
Yield:496062-15-8 40%
Reaction Conditions:
with copper(ll) bromide in acetonitrile at -20 - 15; for 2 h;
Steps:
172 Example 172 Preparation of methyl (2-bromo-5-methyl-1,3-thiazol-4-yl)acetate
To a solution of CuBr2 (4.03 g, 18.1 mmol) and t-butyl nitrite (2.82 mL, 23.8 mmol) in MeCN (210 mL) was added the compound of Example 170 (2.95 g, 15.9 mmol) at -20° C.
The reaction mixture was slowly warmed to 15° C., at which point the evolution of N2 was observed.
After stirring for an additional 2 hours at 15° C., the reaction mixture was diluted with Et2O (400 mL) and washed with a 10% solution of HCl (200 mL).
The solvent layers were separated, the aqueous re-extracted with Et2O (2*300 mL), and the combined organic layers dried (MgSO4), filtered, and concentrated under reduced pressure.
The residue was then purified by silica gel flash chromatography (98:2, hexanes/EtOAc) to afford bromide Example 172 (1.6 g, 40%) as a colorless oil that solidifies upon standing. (C7H8BrNO2S): LC-MS, RT 2.56 min., M+H 250.3; 1H NMR (CDCl3): δ 2.26 (s, 3H), 3.60 (s, 2H), 3.61 (s, 3H).
References:
US2018/153860,2018,A1 Location in patent:Paragraph 0553
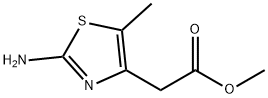
259654-73-4
40 suppliers
$13.00/250mg

496062-15-8
12 suppliers
inquiry
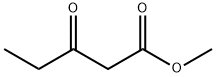
30414-53-0
313 suppliers
$5.00/1g

496062-15-8
12 suppliers
inquiry

105983-77-5
43 suppliers
$130.50/1g

496062-15-8
12 suppliers
inquiry