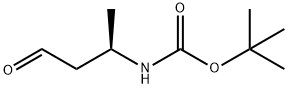
Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:497861-77-5
- Molecular formula:C9H17NO3
- Molecular Weight:187.24
![Carbamic acid, N-[(1R)-3-(methoxymethylamino)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/842103-83-7.gif)
842103-83-7
0 suppliers
inquiry
![Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/497861-77-5.gif)
497861-77-5
27 suppliers
$52.00/25mg
Yield:497861-77-5 84%
Reaction Conditions:
Stage #1: (R)-tert-butyl 4-(methoxy(methyl)amino)-4-oxobutan-2-ylcarbamatewith lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at -5 - 0; for 1.5 h;
Stage #2: with potassium hydrogensulfate in tetrahydrofuran;diethyl ether;water;
Steps:
72.C
C: (R)-tert-Butyl 4-oxobutan-2-ylcarbamate [00346] To a solution of (R)-tert-butyl 4-(methoxy(methyl)amino)-4-oxobutan-2- ylcarbamate (2.48 g, 10.1 mmol) in Et20 (25 niL) cooled to -5 °C was added dropwise a 2.0 M solution of LAH in THF (6.28 niL, 12.6 mmol). After the addition, the resulting slightly milky solution was stirred at 0 °C for 1.5 hr. The reaction mixture was quenched with 1 M aqueous KHSO4 (20 mL) and diluted with water (30 mL). The resulting solution with white milky precipitate was extracted with Et20 (3x). The combined Et20 extracts were washed with 0.5 M aqueous KHSO4 (2 x 20 mL), saturated aqueous aHC03, water, and saturated aqueous NaCl, dried over Na2S04, filtered and concentrated. The residue was dried under vacuum to give (R)-tert-butyl 4-oxobutan-2-ylcarbamate (1.57 g, 8.40 mmol, 84% yield) as a colorless oil. XH NMR (400 MHz, CDC13) δ ppm 9.76 (s, 1H), 4.64 (s, 1H), 4.20 - 4.08 (m, 1H), 2.69 - 2.52 (m, 2H), 1.43 (s, 9H), 1.24 (d, J = 6.8 Hz, 3H).
References:
WO2012/125622,2012,A1 Location in patent:Page/Page column 137
![Butanoic acid, 3-[[(1,1-dimethylethoxy)carbonyl]amino]-, phenylmethyl ester, (3R)-](/CAS/20210305/GIF/163188-28-1.gif)
163188-28-1
0 suppliers
inquiry
![Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/497861-77-5.gif)
497861-77-5
27 suppliers
$52.00/25mg
![Carbamic acid, [(1R)-3-hydroxy-1-methylpropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/167216-17-3.gif)
167216-17-3
47 suppliers
inquiry
![Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/497861-77-5.gif)
497861-77-5
27 suppliers
$52.00/25mg
159877-47-1
64 suppliers
$37.00/250mg
![Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/497861-77-5.gif)
497861-77-5
27 suppliers
$52.00/25mg

24424-99-5
859 suppliers
$13.50/25G
![Carbamic acid, [(1R)-1-methyl-3-oxopropyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/497861-77-5.gif)
497861-77-5
27 suppliers
$52.00/25mg