
4H-1-Benzopyran-4-one, 6-ethyl-2,3-dihydro- synthesis
- Product Name:4H-1-Benzopyran-4-one, 6-ethyl-2,3-dihydro-
- CAS Number:672904-14-2
- Molecular formula:C11H12O2
- Molecular Weight:176.21
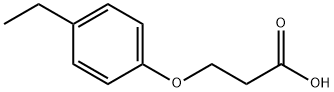
358351-16-3
10 suppliers
inquiry

672904-14-2
22 suppliers
inquiry
Yield:672904-14-2 79%
Reaction Conditions:
Stage #1: 3-(4-ethyl-phenoxy)-propionic acidwith thionyl chloride for 2 h;Heating / reflux;
Stage #2: with aluminum (III) chloride in dichloromethane; for 14 h;Heating / reflux;
Stage #3: with hydrogenchloride in dichloromethane;water;
Steps:
79.3
The carboxylic acid (0.800 g, 4.12 mmol) was stirred with thionyl chloride (6 mL, 82.4 mmol, 20 equiv) at reflux for 2 h. Excess thionyl chloride was removed under reduced pressure. The acid chloride thus obtained was used without further purification in the next reaction. Aluminum chloride (1.10 g, 8.24 mmol, 2 equiv) was added in one portion to a solution of acid chloride as above in dry methylene chloride (50 mL) and the resulting brown mixture was stirred at reflux for 14 h and cooled to room temperature. The mixture was poured onto crushed ice, then 6 M hydrochloric acid (20 mL) was added and the mixture was extracted with methylene chloride. The combined organics were washed with saturated sodium chloride, dried (magnesium sulfate), and concentrated under reduced pressure. Purification by flash column chromatography (silica, gradient 10: 1, and 6: 1 hexanes/ethyl acetate) yielded chromonone 37 (574 mg, 79%) as a colorless oil :'H NMR (300 MHz, CDCl3) 5 7.72 (d, J=2. 2 Hz, 1H), 7.32 (dd, J=8. 5,2. 2 Hz, 1H), 6.90 (d, J=8. 5 Hz, 1H), 4.52 (t, J=6. 5 Hz, 2H), 2,80 (t, J=6. 5 Hz, 2H), 2.60 (q, J=7. 6 Hz, 2H), 1.20 (t, J=7. 6 Hz, 3H); ESI MS m/z 177 [C11 H1202+H]
References:
WO2005/87714,2005,A2 Location in patent:Page/Page column 274; 276
24539-92-2
54 suppliers
$49.00/5g

672904-14-2
22 suppliers
inquiry

259532-90-6
1 suppliers
inquiry

672904-14-2
22 suppliers
inquiry