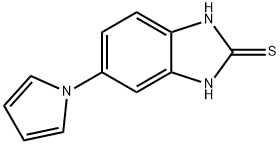
5-(1H-Pyrrol-1-yl)-1H-benzo[d]imidazole-2-thiol synthesis
- Product Name:5-(1H-Pyrrol-1-yl)-1H-benzo[d]imidazole-2-thiol
- CAS Number:172152-53-3
- Molecular formula:C11H9N3S
- Molecular Weight:215.27
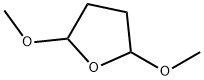
696-59-3
407 suppliers
$7.00/25g
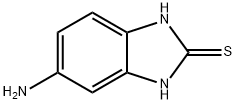
2818-66-8
226 suppliers
$50.00/10g
![5-(1H-Pyrrol-1-yl)-1H-benzo[d]imidazole-2-thiol](/CAS/GIF/172152-53-3.gif)
172152-53-3
202 suppliers
inquiry
Yield:172152-53-3 85%
Reaction Conditions:
with sodium acetate in acetic acid at 50; for 4 h;Product distribution / selectivity;
Steps:
1
[48] Water (100ml) and anhydrous sodium acetate (16.02g, 0.20mole) were added, and then 2-mercapto-5- aminobenzimidazole (32.27g, 0.20mole),2,5-dimethoxytetrahydrofuran (28.4g, 0.21mole), and acetic acid (100ml) were added. Then, stirring was carried out at 5O0C for 4 hours. The resultant product was cooled to 50C and tetrahydrofuran (420ml) was added thereto. The mixture was neutralized with a sodium hydroxide aqueous solution, and water (130ml) was added to carry out layer- separation. Then, an organic layer was washed with a sodium hydroxide aqueous solution. The organic layer was dried by anhydrous magnesium sulfate and concentrated, and then crystallized by ethyl acetate and n-hexane to provide a final compound, that is, 5-(lH-pyrrol-l-yl)-2-mercaptobenzimidazole represented by Formula 3. Then, the obtained compound was confirmed.[49] M.P. 311.80C. Direct inlet MS (EI) for C H N S m/z (relative intensity) 215 (M+,100)[50] 1H NMR (200MHz, DMSO) δ 6.22 (t, 2H), 7.19 (m, 3H), 7.25 (t, 2H), 12.46 (b, IH)[51] Yield: 35.7g (85%)
References:
WO2009/151189,2009,A1 Location in patent:Page 5-6
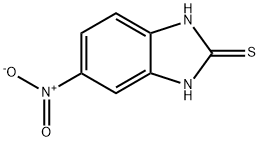
6325-91-3
194 suppliers
$18.00/1g
![5-(1H-Pyrrol-1-yl)-1H-benzo[d]imidazole-2-thiol](/CAS/GIF/172152-53-3.gif)
172152-53-3
202 suppliers
inquiry