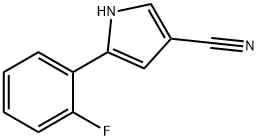
5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile synthesis
- Product Name:5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
- CAS Number:1240948-77-9
- Molecular formula:C11H7FN2
- Molecular Weight:186.19
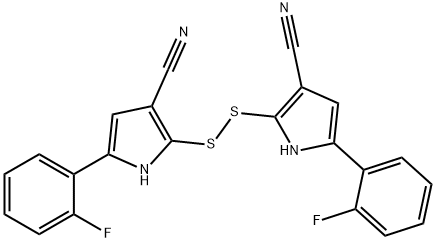
1240948-83-7

1240948-77-9
To a 100 mL four-necked flask under nitrogen protection was added nickel ryenne (12.6 g), N,N-dimethylacetamide (30 mL), morpholine (1.36 mL, 15.6 mmol), and 2,2'-dithiobis[5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile] (4.50 g, 10.4 mmol) to a 15 mL solution of N,N-dimethylacetamide (15 mL) solution. The reaction mixture was heated at reflux at 105 °C for 5.5 hours. After completion of the reaction, the mixture was cooled, the catalyst was removed by filtration, and the filter cake was washed sequentially with N,N-dimethylacetamide and ethyl acetate. Liquid-liquid partitioning was carried out by adding 5% brine to the filtrate and washing solution. The aqueous layer was extracted three times with ethyl acetate. The organic layers were combined, washed with 5% brine and concentrated to dryness under reduced pressure. Ethanol (22.5 mL) was added to the residue and heated to dissolve. Water (45 mL) was added to induce crystallization. The slurry was heated to reflux for 1 hour, then cooled to room temperature and an ice-water mixture was added. Crystals were collected by filtration and washed with an ice-cooled water/ethanol (1:2, 10 mL) mixture. The crystals were dried under reduced pressure at 50 °C to afford the title compound 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (3.45 g, 85% yield). The product was confirmed by 1H-NMR (DMSO-d6, TMS, 300 MHz), mass spectrometry (EI) and elemental analysis.
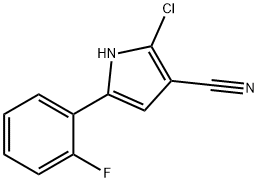
1240948-72-4
124 suppliers
inquiry

1240948-77-9
250 suppliers
inquiry
Yield:1240948-77-9 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20;
Steps:
4 Preparation of 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (Intermediate V-1)
2 g of the intermediate IV-1 was dissolved in 40 ml of methanol, 200 mg of 10% palladium on carbon was added, hydrogen gas was introduced, and the reaction was stirred at room temperature overnight. After the reaction was completed, it was suction filtered with a celite funnel containing celite, and the filtrate was concentrated to give compound of formula V-1, 1.65 g of white solid, yield 98%
References:
CN108558831,2018,A Location in patent:Paragraph 0156-0159

1240948-83-7
0 suppliers
inquiry

1240948-77-9
250 suppliers
inquiry
![Benzenecarbothioic acid, S-[3-cyano-5-(2-fluorophenyl)-1H-pyrrol-2-yl] ester](/CAS/20210305/GIF/1240948-81-5.gif)
1240948-81-5
0 suppliers
inquiry

1240948-77-9
250 suppliers
inquiry
![2-[2-(2-Fluorophenyl)-2-oxoethyl]propanedinitrile](/CAS/20150408/GIF/312307-38-3.gif)
312307-38-3
163 suppliers
inquiry

1240948-77-9
250 suppliers
inquiry
![2-[2-(2-Fluorophenyl)-2-oxoethyl]propanedinitrile](/CAS/20150408/GIF/312307-38-3.gif)
312307-38-3
163 suppliers
inquiry
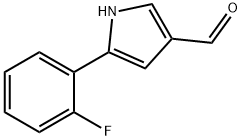
881674-56-2
470 suppliers
$6.00/250mg

1240948-77-9
250 suppliers
inquiry